MAC Family Transcription Factors Enhance the Tolerance of Mycelia to Heat Stress and Promote the Primordial Formation Rate of Pleurotus ostreatus
- PMID: 38248923
- PMCID: PMC10816978
- DOI: 10.3390/jof10010013
MAC Family Transcription Factors Enhance the Tolerance of Mycelia to Heat Stress and Promote the Primordial Formation Rate of Pleurotus ostreatus
Abstract
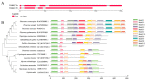
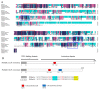
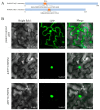
Pleurotus ostreatus is a typical tetrapolar heterologous edible mushroom, and its growth and development regulatory mechanism has become a research hotspot in recent years. The MAC1 protein is a transcription factor that perceives copper and can regulate the expression of multiple genes, thereby affecting the growth and development of organisms. However, its function in edible mushrooms is still unknown. In this study, two transcription factor genes, PoMCA1a and PoMAC1b, were identified. Afterwards, PoMAC1 overexpression (OE) and RNA interference (RNAi) strains were constructed to further explore gene function. The results showed that the PoMAC1 mutation had no significant effect on the growth rate of mycelia. Further research has shown that OE-PoMAC1a strains and RNAi-PoMAC1b strains exhibit strong tolerance under 32 °C heat stress. However, under 40 °C heat stress, the OE of PoMAC1a and PoMAC1b promoted the recovery of mycelial growth after heat stress. Second, the OE of PoMAC1a can promote the rapid formation of primordia and shorten the cultivation cycle. In summary, this study indicated that there are functional differences between PoMAC1a and PoMAC1b under different heat stresses during the vegetative growth stage, and PoMAC1a has a positive regulatory effect on the formation of primordia during the reproductive growth stage.
Keywords: Oyster mushroom; fruiting body; gene overexpression; gene silencing; stress tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Xia F., Fan J., Zhu M., Tong H. Antioxidant Effects of a Water-Soluble Proteoglycan Isolated from the Fruiting Bodies of Pleurotus ostreatus. J. Taiwan Inst. Chem. Eng. 2011;42:402–407. doi: 10.1016/j.jtice.2010.08.012. - DOI
-
- BBarbosa J.R., Freitas M.M.S., Oliveira L.C., Martins L.H.S., Almada-Vilhena A.O., Oliveira R.M., Pieczarka J.C., Davi do Socorro B., Junior R.N.C. Obtaining Extracts Rich in Antioxidant Polysaccharides from the Edible Mushroom Pleurotus ostreatus Using Binary System with Hot Water and Supercritical CO2. Food Chem. 2020;330:127173. doi: 10.1016/j.foodchem.2020.127173. - DOI - PubMed
-
- Othman A., Elshafei A.M., Hassan M.M., Haroun B.M., Farrag A.A. Purification, Biochemical Characterization and Applications of Pleurotus ostreatus ARC280 Laccase. Br. Microbiol. Res. J. 2014;4:1418–1439. doi: 10.9734/BMRJ/2014/11218. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

