Design of a Multi-Epitope Vaccine against Histoplasma capsulatum through Immunoinformatics Approaches
- PMID: 38248954
- PMCID: PMC10817582
- DOI: 10.3390/jof10010043
Design of a Multi-Epitope Vaccine against Histoplasma capsulatum through Immunoinformatics Approaches
Abstract
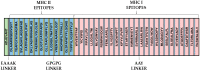
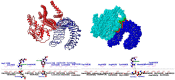
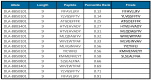
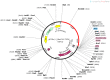
Histoplasmosis is a widespread systemic disease caused by Histoplasma capsulatum, prevalent in the Americas. Despite its significant morbidity and mortality rates, no vaccines are currently available. Previously, five vaccine targets and specific epitopes for H. capsulatum were identified. Immunoinformatics has emerged as a novel approach for determining the main immunogenic components of antigens through in silico methods. Therefore, we predicted the main helper and cytotoxic T lymphocytes and B-cell epitopes for these targets to create a potential multi-epitope vaccine known as HistoVAC-TSFM. A total of 38 epitopes were found: 23 common to CTL and B-cell responses, 11 linked to HTL and B cells, and 4 previously validated epitopes associated with the B subunit of cholera toxin, a potent adjuvant. In silico evaluations confirmed the stability, non-toxicity, non-allergenicity, and non-homology of these vaccines with the host. Notably, the vaccine exhibited the potential to trigger both innate and adaptive immune responses, likely involving the TLR4 pathway, as supported by 3D modeling and molecular docking. The designed HistoVAC-TSFM appears promising against Histoplasma, with the ability to induce important cytokines, such as IFN-γ, TNF-α, IL17, and IL6. Future studies could be carried out to test the vaccine's efficacy in in vivo models.
Keywords: epizootic lymphangitis; fungi vaccine; histoplasmosis; immunoinformatic; multi-epitope.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources

