Hyperpolarisation of Mitochondrial Membranes Is a Critical Component of the Antifungal Mechanism of the Plant Defensin, Ppdef1
- PMID: 38248963
- PMCID: PMC10817573
- DOI: 10.3390/jof10010054
Hyperpolarisation of Mitochondrial Membranes Is a Critical Component of the Antifungal Mechanism of the Plant Defensin, Ppdef1
Abstract
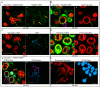
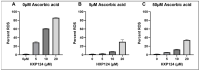
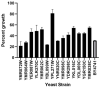
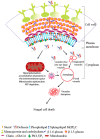
Plant defensins are a large family of small cationic proteins with diverse functions and mechanisms of action, most of which assert antifungal activity against a broad spectrum of fungi. The partial mechanism of action has been resolved for a small number of members of plant defensins, and studies have revealed that many act by more than one mechanism. The plant defensin Ppdef1 has a unique sequence and long loop 5 with fungicidal activity against a range of human fungal pathogens, but little is known about its mechanism of action. We screened the S. cerevisiae non-essential gene deletion library and identified the involvement of the mitochondria in the mechanism of action of Ppdef1. Further analysis revealed that the hyperpolarisation of the mitochondrial membrane potential (MMP) activates ROS production, vacuolar fusion and cell death and is an important step in the mechanism of action of Ppdef1, and it is likely that a similar mechanism acts in Trichophyton rubrum.
Keywords: Ppdef1; S. cerevisiae; Trichophyton rubrum; antifungal; hyperpolarisation; plant defensin.
Conflict of interest statement
N.L.v.d.W., J.A.M. and M.A.A. are Hexima Ltd. shareholders. N.L.v.d.W. and M.A.A. are inventors of the method for the treatment of fungal infections, EP3209319B1 and US9713632B2. Hexima Ltd. has granted permission to publish the results. We confirm that neither the manuscript nor any parts of its content are currently under consideration or published in another journal. Authors K.P., J.A.M., R.L., K.S.H., R.G., E.L., N.L.v.d.W., M.R.B. and M.A.A. were employed by the company Hexima Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

