The Metabolic Regulation of Amino Acid Synthesis Counteracts Reactive Nitrogen Stress via Aspergillus nidulans Cross-Pathway Control
- PMID: 38248967
- PMCID: PMC10817288
- DOI: 10.3390/jof10010058
The Metabolic Regulation of Amino Acid Synthesis Counteracts Reactive Nitrogen Stress via Aspergillus nidulans Cross-Pathway Control
Abstract
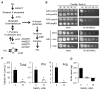
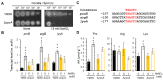
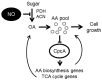
Nitric oxide (NO) is a natural reactive nitrogen species (RNS) that alters proteins, DNA, and lipids and damages biological activities. Although microorganisms respond to and detoxify NO, the regulation of the cellular metabolic mechanisms that cause cells to tolerate RNS toxicity is not completely understood. We found that the proline and arginine auxotrophic proA5 and argB2 mutants of the fungus Aspergillus nidulans require more arginine and proline for normal growth under RNS stress that starves cells by accumulating fewer amino acids. Fungal transcriptomes indicated that RNS stress upregulates the expression of the biosynthetic genes required for global amino acids, including proline and arginine. A mutant of the gene disruptant, cpcA, which encodes the transcriptional regulation of the cross-pathway control of general amino acid synthesis, did not induce these genes, and cells accumulated fewer amino acids under RNS stress. These results indicated a novel function of CpcA in the cellular response to RNS stress, which is mediated through amino acid starvation and induces the transcription of genes for general amino acid synthesis. Since CpcA also controls organic acid biosynthesis, impaired intermediates of such biosynthesis might starve cells of amino acids. These findings revealed the importance of the mechanism regulating amino acid homeostasis for fungal responses to and survival under RNS stress.
Keywords: amino acid; nitric oxide; reactive species; starvation; stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

