Molecular Signaling and Metabolic Responses during the Interaction between Human Keratinocytes (HaCaT) and the Dermatophyte Trichophyton rubrum
- PMID: 38248981
- PMCID: PMC10820588
- DOI: 10.3390/jof10010072
Molecular Signaling and Metabolic Responses during the Interaction between Human Keratinocytes (HaCaT) and the Dermatophyte Trichophyton rubrum
Abstract
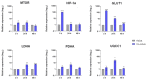
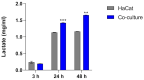
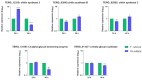
Trichophyton rubrum is the leading causative agent of dermatophytosis worldwide. Keratinocytes are the first line of defense that drives an immune response against fungal invasion. Host-specific pattern recognition receptors (PRRs) recognize pathogen-associated molecular patterns (PAMPs) to trigger immunological pathways. Fungal cell wall components are the primary sources of fungal PAMPs, and some pathogens increase cell wall rearrangement to evade the immune system. Glycolysis and enhanced lactate levels are critical for improving host immune responses to fungal infections. Using reverse transcription-quantitative polymerase chain reaction (RT-qPCR), we evaluated the transcriptional responses of human genes involved in fungal recognition and glycolytic metabolism and fungal cell-wall-related genes in a co-culture model of human keratinocytes with T. rubrum. We observed the upregulation of several Toll-like receptors (TLRs), NOD-like receptors (NLRs), and glycolytic genes. Complementarily, we measured intra- and extracellular glucose levels and the increase in lactate production in the co-culture supernatant. We noted a distinct transcriptional regulation pattern of fungal cell-wall-related genes from fungal growth on keratin as the primary carbon source compared to co-culture with human keratinocytes. Our results showed new insights into the transcriptional adaptation of keratinocytes, particularly in regulating genes involved in sensing and metabolic processes, during the interaction with T. rubrum.
Keywords: PRRs; Trichophyton rubrum; cell wall; glucose; immune response; keratinocytes; lactate; pathogen–host interaction.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures





References
-
- Cruz A.H.S., Santos R.S., Martins M.P., Peres N.T.A., Trevisan G.L., Mendes N.S., Martinez-Rossi N.M., Rossi A. Relevance of nutrient-sensing in the pathogenesis of Trichophyton rubrum and Trichophyton interdigitale. Front. Fungal Biol. 2022;3:858968. doi: 10.3389/ffunb.2022.858968. - DOI - PMC - PubMed
-
- Martins-Santana L., Petrucelli M.F., Sanches P.R., Martinez-Rossi N.M., Rossi A. Peptidase regulation in Trichophyton rubrum is mediated by the synergism between alternative splicing and StuA-dependent transcriptional mechanisms. Front. Microbiol. 2022;13:2251. doi: 10.3389/fmicb.2022.930398. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

