Decomposition Technique for Bio-Transmittance Imaging Based on Attenuation Coefficient Matrix Inverse
- PMID: 38249007
- PMCID: PMC10817561
- DOI: 10.3390/jimaging10010022
Decomposition Technique for Bio-Transmittance Imaging Based on Attenuation Coefficient Matrix Inverse
Abstract
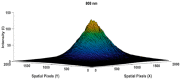
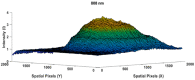
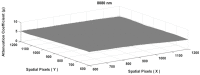
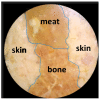
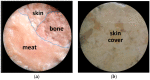
Human body tissue disease diagnosis will become more accurate if transmittance images, such as X-ray images, are separated according to each constituent tissue. This research proposes a new image decomposition technique based on the matrix inverse method for biological tissue images. The fundamental idea of this research is based on the fact that when k different monochromatic lights penetrate a biological tissue, they will experience different attenuation coefficients. Furthermore, the same happens when monochromatic light penetrates k different biological tissues, as they will also experience different attenuation coefficients. The various attenuation coefficients are arranged into a unique k×k-dimensional square matrix. k-many images taken by k-many different monochromatic lights are then merged into an image vector entity; further, a matrix inverse operation is performed on the merged image, producing N-many tissue thickness images of the constituent tissues. This research demonstrates that the proposed method effectively decomposes images of biological objects into separate images, each showing the thickness distributions of different constituent tissues. In the future, this proposed new technique is expected to contribute to supporting medical imaging analysis.
Keywords: attenuation coefficient; biological tissue; image decomposition technique; matrix inverse; monochromatic light; near-infrared; transmittance image.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Thomson J.J. The Röntgen Rays. Nature. 1896;53:581–583. doi: 10.1038/053581e0. - DOI
-
- Jaszczak R.J., Coleman R.E., Lim C.B. SPECT: Single Photon Emission Computed Tomography. IEEE Trans. Nucl. Sci. 1980;27:1137–1153. doi: 10.1109/TNS.1980.4330986. - DOI
-
- Dorbala S., Ananthasubramaniam K., Armstrong I.S., Chareonthaitawee P., DePuey E.G., Einstein A.J., Gropler R.J., Holly T.A., Mahmarian J.J., Park M.-A., et al. Single Photon Emission Computed Tomography (SPECT) Myocardial Perfusion Imaging Guidelines: Instrumentation, Acquisition, Processing, and Interpretation. J. Nucl. Cardiol. 2018;25:1784–1846. doi: 10.1007/s12350-018-1283-y. - DOI - PubMed
-
- Maisey M.N. Positron Emission Tomography in Clinical Medicine. In: Bailey D.L., Townsend D.W., Valk P.E., Maisey M.N., editors. Positron Emission Tomography: Basic Sciences. Springer; London, UK: 2005. pp. 1–12. - DOI
-
- Hashemi R.H., Bradley W.G., Jr., Lisanti C.J. MRI: The Basics. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2012. pp. 1–385.
Grants and funding
LinkOut - more resources
Full Text Sources

