Use of the urine Determine LAM test in the context of tuberculosis diagnosis among inpatients with HIV in Ghana: a mixed methods study
- PMID: 38249371
- PMCID: PMC10797072
- DOI: 10.3389/fpubh.2023.1271763
Use of the urine Determine LAM test in the context of tuberculosis diagnosis among inpatients with HIV in Ghana: a mixed methods study
Abstract
Background: The urine Determine LAM test has the potential to identify tuberculosis (TB) and reduce early mortality among people living with HIV. However, implementation of the test in practice has been slow. We aimed to understand how a Determine LAM intervention was received and worked in a Ghanaian in-hospital context.
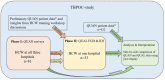
Design/methods: Nested in a Determine LAM intervention study, we conducted a two-phase explanatory sequential mixed methods study at three hospitals in Ghana between January 2021 and January 2022. We performed a quantitative survey with 81 healthcare workers (HCWs), four qualitative focus-group discussions with 18 HCWs, and 15 in-depth HCW interviews. Integration was performed at the methods and analysis level. Descriptive analysis, qualitative directed content analysis, and mixed methods joint display were used.
Results: The gap in access to TB testing when relying on sputum GeneXpert MTB/Rif alone was explained by difficulties in obtaining sputum samples and an in-hospital system that relies on relatives. The Determine LAM test procedure was experienced as easy, and most eligible patients received a test. HCWs expressed that immediate access to Determine LAM tests empowered them in rapid diagnosis. The HCW survey confirmed that bedside was the most common place for Determine LAM testing, but qualitative interviews with nurses revealed concerns about patient confidentiality when performing and disclosing the test results at the bedside. Less than half of Determine LAM-positive patients were initiated on TB treatment, and qualitative data identified a weak link in the communication of the Determine LAM results. Moreover, HCWs were reluctant to initiate Determine LAM-positive patients on TB treatment due to test specificity concerns. The Determine LAM intervention did not have an impact on the time to TB treatment as expected, but patients were, in general, initiated on TB treatment rapidly. We further identified a barrier to accessing TB treatment during weekends and that treatment by tradition is administrated early in the morning.
Conclusion: The Determine LAM testing was feasible and empowered HCWs in the management of HIV-associated TB. Important gaps in routine care and Determine LAM-enhanced TB care were often explained by the context. These findings may inform in-hospital quality improvement work and scale-up of Determine LAM in similar settings.
Keywords: Determine LAM; HIV; Xpert MTB/RIF; diagnosis; mixed methods; qualitative interviews; tuberculosis.
Copyright © 2024 Åhsberg, Tersbøl, Puplampu, Kwashie, Commey, Adusi-Poku, Moseholm, Andersen, Kenu, Lartey, Johansen and Bjerrum.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Lawn SD, Kerkhoff AD, Burton R, Schutz C, Boulle A, Vogt M, et al. Diagnostic accuracy, incremental yield and prognostic value of determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med. (2017) 15:67. doi: 10.1186/s12916-017-0822-8, PMID: - DOI - PMC - PubMed
-
- Gupta-Wright A, Corbett EL, van Oosterhout JJ, Wilson D, Grint D, Alufandika-Moyo M, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet. (2018) 392:292–301. doi: 10.1016/S0140-6736(18)31267-4, PMID: - DOI - PMC - PubMed
-
- World Health Organization . WHO consolidated guidelines on tuberculosis. Module 2: screening – systematic screening for tuberculosis disease. Geneva: World Health Organization; (2021). - PubMed
-
- World Health Organization . Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV: policy update 2019. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; (2019).
-
- World Health Organization . The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV. Policy update. Geneva: WHO; (2015).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

