Enhancing the mechanical strength of 3D printed GelMA for soft tissue engineering applications
- PMID: 38249436
- PMCID: PMC10797197
- DOI: 10.1016/j.mtbio.2023.100939
Enhancing the mechanical strength of 3D printed GelMA for soft tissue engineering applications
Abstract
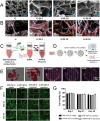
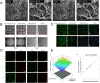
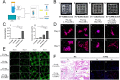
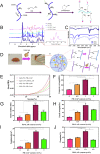
Gelatin methacrylate (GelMA) hydrogels have gained significant traction in diverse tissue engineering applications through the utilization of 3D printing technology. As an artificial hydrogel possessing remarkable processability, GelMA has emerged as a pioneering material in the advancement of tissue engineering due to its exceptional biocompatibility and degradability. The integration of 3D printing technology facilitates the precise arrangement of cells and hydrogel materials, thereby enabling the creation of in vitro models that simulate artificial tissues suitable for transplantation. Consequently, the potential applications of GelMA in tissue engineering are further expanded. In tissue engineering applications, the mechanical properties of GelMA are often modified to overcome the hydrogel material's inherent mechanical strength limitations. This review provides a comprehensive overview of recent advancements in enhancing the mechanical properties of GelMA at the monomer, micron, and nano scales. Additionally, the diverse applications of GelMA in soft tissue engineering via 3D printing are emphasized. Furthermore, the potential opportunities and obstacles that GelMA may encounter in the field of tissue engineering are discussed. It is our contention that through ongoing technological progress, GelMA hydrogels with enhanced mechanical strength can be successfully fabricated, leading to the production of superior biological scaffolds with increased efficacy for tissue engineering purposes.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Cited by
-
Omnidirectional anisotropic embedded 3D bioprinting.Mater Today Bio. 2024 Jul 17;27:101160. doi: 10.1016/j.mtbio.2024.101160. eCollection 2024 Aug. Mater Today Bio. 2024. PMID: 39155942 Free PMC article.
-
Enhancing angiogenesis in peri-implant soft tissue with bioactive silk fibroin microgroove coatings on zirconia surfaces.Regen Biomater. 2024 Jun 17;11:rbae068. doi: 10.1093/rb/rbae068. eCollection 2024. Regen Biomater. 2024. PMID: 39027360 Free PMC article.
-
Advances of naturally derived biomedical polymers in tissue engineering.Front Chem. 2024 Nov 20;12:1469183. doi: 10.3389/fchem.2024.1469183. eCollection 2024. Front Chem. 2024. PMID: 39635576 Free PMC article. Review.
-
Designing Porosity-Tailored Hydrogel Sponges with Controlled Cell Positioning Using Dispersible, Autofragmented Sacrificial Microfibers.ACS Omega. 2025 Feb 10;10(12):11900-11910. doi: 10.1021/acsomega.4c08536. eCollection 2025 Apr 1. ACS Omega. 2025. PMID: 40191313 Free PMC article.
-
Bioprinting of GelMA-Based Hydrogels to Aid in Creation of Biomimetic 3D Models for Glioblastoma.Micromachines (Basel). 2025 May 29;16(6):654. doi: 10.3390/mi16060654. Micromachines (Basel). 2025. PMID: 40572374 Free PMC article.
References
-
- Zhong H., Huang J., Wu J., Du J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res. 2022;15:787–804. doi: 10.1007/s12274-021-3593-7. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

