Influence of perinatal and childhood exposure to tobacco and mercury in children's gut microbiota
- PMID: 38249448
- PMCID: PMC10799562
- DOI: 10.3389/fmicb.2023.1258988
Influence of perinatal and childhood exposure to tobacco and mercury in children's gut microbiota
Abstract
Background: Early life determinants of the development of gut microbiome composition in infants have been widely investigated; however, if early life pollutant exposures, such as tobacco or mercury, have a persistent influence on the gut microbial community, its stabilization at later childhood remains largely unknown.
Objective: In this exposome-wide study, we aimed at identifying the contribution of exposure to tobacco and mercury from the prenatal period to childhood, to individual differences in the fecal microbiome composition of 7-year-old children, considering co-exposure to a width of established lifestyle and clinical determinants.
Methods: Gut microbiome was studied by 16S rRNA amplicon sequencing in 151 children at the genus level. Exposure to tobacco was quantified during pregnancy through questionnaire (active tobacco consumption, second-hand smoking -SHS) and biomonitoring (urinary cotinine) at 4 years (urinary cotinine, SHS) and 7 years (SHS). Exposure to mercury was quantified during pregnancy (cord blood) and at 4 years (hair). Forty nine other potential environmental determinants (12 at pregnancy/birth/infancy, 15 at 4 years and 22 at 7 years, such as diet, demographics, quality of living/social environment, and clinical records) were registered. We used multiple models to determine microbiome associations with pollutants including multi-determinant multivariate analysis of variance and linear correlations (wUnifrac, Bray-Curtis and Aitchison ß-diversity distances), single-pollutant permutational multivariate analysis of variance adjusting for co-variates (Aitchison), and multivariable association model with single taxa (MaAsLin2; genus). Sensitivity analysis was performed including genetic data in a subset of 107 children.
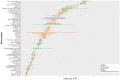
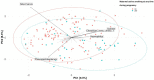
Results: Active smoking in pregnancy was systematically associated with microbiome composition and ß-diversity (R2 2-4%, p < 0.05, Aitchison), independently of other co-determinants. However, in the adjusted single pollutant models (PERMANOVA), we did not find any significant association. An increased relative abundance of Dorea and decreased relative abundance of Akkermansia were associated with smoking during pregnancy (q < 0.05).
Discussion: Our findings suggest a long-term sustainable effect of prenatal tobacco exposure on the children's gut microbiota. This effect was not found for mercury exposure or tobacco exposure during childhood. Assessing the role of these exposures on the children's microbiota, considering multiple environmental factors, should be further investigated.
Keywords: 16S rRNA; birth cohort; children; diet; gut microbiota; mercury; second-hand smoke; smoking during pregnancy.
Copyright © 2024 Pérez-Castro, D’Auria, Llambrich, Fernández-Barrés, Lopez-Espinosa, Llop, Regueiro, Bustamante, Francino, Vrijheid and Maitre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Perinatal Exposure to Tobacco Smoke and Its Association with the Maternal and Offspring Microbiome: A Systematic Review.Healthcare (Basel). 2024 Sep 19;12(18):1874. doi: 10.3390/healthcare12181874. Healthcare (Basel). 2024. PMID: 39337215 Free PMC article. Review.
-
Associations of Childhood and Perinatal Blood Metals with Children's Gut Microbiomes in a Canadian Gestation Cohort.Environ Health Perspect. 2022 Jan;130(1):17007. doi: 10.1289/EHP9674. Epub 2022 Jan 17. Environ Health Perspect. 2022. PMID: 35037767 Free PMC article.
-
Early life tobacco exposure and children's telomere length: The HELIX project.Sci Total Environ. 2020 Apr 1;711:135028. doi: 10.1016/j.scitotenv.2019.135028. Epub 2019 Nov 20. Sci Total Environ. 2020. PMID: 32000334
-
Prenatal environmental tobacco smoke exposure and early childhood body mass index.Paediatr Perinat Epidemiol. 2010 Nov;24(6):524-34. doi: 10.1111/j.1365-3016.2010.01146.x. Epub 2010 Aug 16. Paediatr Perinat Epidemiol. 2010. PMID: 20955230 Free PMC article.
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
Cited by
-
Biomonitoring of Mercury and Lead Levels in the Blood of Children Living near a Tropical River Impacted by Artisanal and Small-Scale Gold Mining in Colombia.Toxics. 2024 Oct 13;12(10):744. doi: 10.3390/toxics12100744. Toxics. 2024. PMID: 39453164 Free PMC article.
-
Perinatal Exposure to Tobacco Smoke and Its Association with the Maternal and Offspring Microbiome: A Systematic Review.Healthcare (Basel). 2024 Sep 19;12(18):1874. doi: 10.3390/healthcare12181874. Healthcare (Basel). 2024. PMID: 39337215 Free PMC article. Review.
References
-
- Aurrekoetxea J. J., Murcia M., Rebagliato M., Guxens M., Fernández-Somoano A., López M. J., et al. (2016). Second-hand smoke exposure in 4-year-old children in Spain: sources, associated factors and urinary cotinine. Environ. Res. 145, 116–125. doi: 10.1016/j.envres.2015.11.028, PMID: - DOI - PubMed
-
- Aurrekoetxea J. J., Murcia M., Rebagliato M., López M. J., Castilla A. M., Santa-Marina L., et al. (2013). Determinants of self-reported smoking and misclassification during pregnancy, and analysis of optimal cut-off points for urinary cotinine: a cross-sectional study. BMJ Open 3:e002034. doi: 10.1136/bmjopen-2012-002034, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

