Regulation of organic acid and hydrogen production by NADH/NAD+ ratio in Synechocystis sp. PCC 6803
- PMID: 38249449
- PMCID: PMC10797119
- DOI: 10.3389/fmicb.2023.1332449
Regulation of organic acid and hydrogen production by NADH/NAD+ ratio in Synechocystis sp. PCC 6803
Abstract
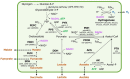
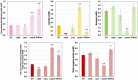
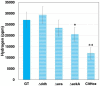
Cyanobacteria serve as useful hosts in the production of substances to support a low-carbon society. Specifically, the unicellular cyanobacterium Synechocystis sp. PCC 6803 (Synechocystis 6803) can produce organic acids, such as acetate, lactate, and succinate, as well as hydrogen, under dark, anaerobic conditions. The efficient production of these compounds appears to be closely linked to the regulation of intracellular redox balance. Notably, alterations in intracellular redox balance have been believed to influence the production of organic acids and hydrogen. To achieve these alterations, genetic manipulations involved overexpressing malate dehydrogenase (MDH), knocking out d-lactate dehydrogenase (DDH), or knocking out acetate kinase (AK), which subsequently modified the quantities and ratios of organic acids and hydrogen under dark, anaerobic conditions. Furthermore, the mutants generated displayed changes in the oxidation of reducing powers and the nicotinamide adenine dinucleotide hydrogen (NADH)/NAD+ ratio when compared to the parental wild-type strain. These findings strongly suggest that intracellular redox balance, especially the NADH/NAD+ ratio, plays a pivotal role in the production of organic acids and hydrogen in Synechocystis 6803.
Keywords: Cyanobacteria; acetate; fermentation; hydrogen; lactate; organic acids; succinate.
Copyright © 2024 Akiyama and Osanai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Anionic metabolite biosynthesis enhanced by potassium under dark, anaerobic conditions in cyanobacteria.Sci Rep. 2016 Aug 31;6:32354. doi: 10.1038/srep32354. Sci Rep. 2016. PMID: 27576448 Free PMC article.
-
Malic Enzyme, not Malate Dehydrogenase, Mainly Oxidizes Malate That Originates from the Tricarboxylic Acid Cycle in Cyanobacteria.mBio. 2022 Dec 20;13(6):e0218722. doi: 10.1128/mbio.02187-22. Epub 2022 Oct 31. mBio. 2022. PMID: 36314837 Free PMC article.
-
Malic Enzyme Facilitates d-Lactate Production through Increased Pyruvate Supply during Anoxic Dark Fermentation in Synechocystis sp. PCC 6803.ACS Synth Biol. 2020 Feb 21;9(2):260-268. doi: 10.1021/acssynbio.9b00281. Epub 2020 Feb 13. ACS Synth Biol. 2020. PMID: 32004431
-
Physiological Significance of NAD Kinases in Cyanobacteria.Front Plant Sci. 2019 Jun 27;10:847. doi: 10.3389/fpls.2019.00847. eCollection 2019. Front Plant Sci. 2019. PMID: 31316540 Free PMC article. Review.
-
NAD+/NADH homeostasis affects metabolic adaptation to hypoxia and secondary metabolite production in filamentous fungi.Biosci Biotechnol Biochem. 2018 Feb;82(2):216-224. doi: 10.1080/09168451.2017.1422972. Epub 2018 Jan 12. Biosci Biotechnol Biochem. 2018. PMID: 29327656 Review.
Cited by
-
CyAbrB2 is a nucleoid-associated protein in Synechocystis controlling hydrogenase expression during fermentation.Elife. 2024 Sep 2;13:RP94245. doi: 10.7554/eLife.94245. Elife. 2024. PMID: 39221912 Free PMC article.
-
Insufficient Acetyl-CoA Pool Restricts the Phototrophic Production of Organic Acids in Model Cyanobacteria.Int J Mol Sci. 2024 Nov 1;25(21):11769. doi: 10.3390/ijms252111769. Int J Mol Sci. 2024. PMID: 39519321 Free PMC article. Review.
-
Outlook on Synthetic Biology-Driven Hydrogen Production: Lessons from Algal Photosynthesis Applied to Cyanobacteria.Energy Fuels. 2025 Mar 11;39(11):4987-5006. doi: 10.1021/acs.energyfuels.4c04772. eCollection 2025 Mar 20. Energy Fuels. 2025. PMID: 40134520 Free PMC article. Review.
References
-
- Angermayr S. A., van der Woude A. D., Correddu D., Kern R., Hagemann M., Hellingwerf K. J. (2016). Chirality matters: synthesis and consumption of the d-enantiomer of lactic acid by Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 82, 1295–1304. doi: 10.1128/AEM.03379-15, PMID: - DOI - PMC - PubMed
-
- Baicha Z., Salar-García M. J., Ortiz-Martínez V. M., Hernández-Fernández F. J., De Los Ríos A. P., Labjar N., et al. . (2016). A critical review on microalgae as an alternative source for bioenergy production: a promising low cost substrate for microbial fuel cells. Fuel Process. Technol. 154, 104–116. doi: 10.1016/j.fuproc.2016.08.017 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

