A vesicular stomatitis virus-based African swine fever vaccine prototype effectively induced robust immune responses in mice following a single-dose immunization
- PMID: 38249478
- PMCID: PMC10797088
- DOI: 10.3389/fmicb.2023.1310333
A vesicular stomatitis virus-based African swine fever vaccine prototype effectively induced robust immune responses in mice following a single-dose immunization
Erratum in
-
Corrigendum: A vesicular stomatitis virus-based African swine fever vaccine prototype effectively induced robust immune responses in mice following a single-dose immunization.Front Microbiol. 2025 Mar 18;16:1585665. doi: 10.3389/fmicb.2025.1585665. eCollection 2025. Front Microbiol. 2025. PMID: 40170920 Free PMC article.
Abstract
Introduction: African swine fever (ASF) is a highly contagious hemorrhagic fever disease in pigs caused by African swine fever virus (ASFV). It is very difficult to control and prevent ASF outbreaks due to the absence of safe and effective vaccines.
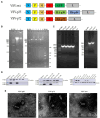
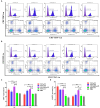
Methods: In order to develop a safe and effective ASF vaccine for the control and prevention of ASF, two ASFV recombinant vesicular stomatitis virus (VSV) live vector vaccine prototypes, containing the gene of p72, and a chimera of p30 and p54, were developed based on the replication-competent VSV, and named VSV-p72 and VSV-p35. The immune potency of VSV-p72 or VSV-p35 alone and in combination was evaluated in BALB/c mice via intramuscular and intranasal vaccination.
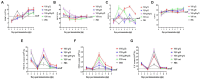
Results: The results indicated that whether administered alone or in combination, the two vaccine prototypes showed acceptable safety in mice and, more importantly, induced high-level specific antibodies against p72, p30, and p54 of ASFV and a strong cellular immune response 28 days after vaccination. The sera from mice vaccinated with the vaccine prototypes significantly inhibited ASFV from infecting porcine alveolar macrophages (PAMs) in vitro. Most notably, the immunized sera from a mixture of VSV-p35 and VSV-p72 inhibited ASFV from infecting PAMs, with an inhibition rate of up to 78.58%.
Conclusion: Overall, our findings suggest that ASFV recombinant VSV live vector vaccine prototypes may become a promising candidate vaccine for the control and prevention of ASF.
Keywords: African swine fever virus; immune potency; safety; vaccine prototypes; vesicular stomatitis virus.
Copyright © 2024 Ma, Shao, Liu, Gao, Peng, Miao, Yang, Hou, Zhou, Qi and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Development and efficacy of a novel mRNA cocktail for the delivery of African swine fever virus antigens and induction of immune responses.Microbiol Spectr. 2025 Jun 3;13(6):e0290924. doi: 10.1128/spectrum.02909-24. Epub 2025 Apr 29. Microbiol Spectr. 2025. PMID: 40298440 Free PMC article.
-
A Non-Hemadsorbing Live-Attenuated Virus Vaccine Candidate Protects Pigs against the Contemporary Pandemic Genotype II African Swine Fever Virus.Viruses. 2024 Aug 19;16(8):1326. doi: 10.3390/v16081326. Viruses. 2024. PMID: 39205300 Free PMC article.
-
Evaluation of the Deletion of MGF110-5L-6L on Swine Virulence from the Pandemic Strain of African Swine Fever Virus and Use as a DIVA Marker in Vaccine Candidate ASFV-G-ΔI177L.J Virol. 2022 Jul 27;96(14):e0059722. doi: 10.1128/jvi.00597-22. Epub 2022 Jul 11. J Virol. 2022. PMID: 35862688 Free PMC article.
-
Recent progress and major gaps in the vaccine development for African swine fever.Braz J Microbiol. 2024 Mar;55(1):997-1010. doi: 10.1007/s42770-024-01264-7. Epub 2024 Feb 5. Braz J Microbiol. 2024. PMID: 38311710 Free PMC article. Review.
-
Regulation of antiviral immune response by African swine fever virus (ASFV).Virol Sin. 2022 Apr;37(2):157-167. doi: 10.1016/j.virs.2022.03.006. Epub 2022 Mar 9. Virol Sin. 2022. PMID: 35278697 Free PMC article. Review.
Cited by
-
Analysis of the Immunogenicity of African Swine Fever F317L Protein and Screening of T Cell Epitopes.Animals (Basel). 2024 Apr 29;14(9):1331. doi: 10.3390/ani14091331. Animals (Basel). 2024. PMID: 38731330 Free PMC article.
-
A Mutant of Africa Swine Fever Virus Protein p72 Enhances Antibody Production and Regulates the Production of Cytokines.Viruses. 2025 Jan 30;17(2):194. doi: 10.3390/v17020194. Viruses. 2025. PMID: 40006949 Free PMC article.
-
Molecular Mechanism of VSV-Vectored ASFV Vaccine Activating Immune Response in DCs.Vet Sci. 2025 Jan 9;12(1):36. doi: 10.3390/vetsci12010036. Vet Sci. 2025. PMID: 39852910 Free PMC article.
References
-
- Abrams C. C., Goatley L., Fishbourne E., Chapman D., Cooke L., Oura C. A., et al. . (2013). Deletion of virulence associated genes from attenuated African swine fever virus isolate OUR T88/3 decreases its ability to protect against challenge with virulent virus. Virology 443, 99–105. doi: 10.1016/j.virol.2013.04.028, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

