LncRNA 51325 Alleviates Bone Cancer Induced Hyperalgesia Through Inhibition of Pum2
- PMID: 38249568
- PMCID: PMC10799577
- DOI: 10.2147/JPR.S446635
LncRNA 51325 Alleviates Bone Cancer Induced Hyperalgesia Through Inhibition of Pum2
Abstract
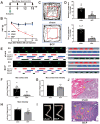
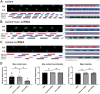
Background: Bone cancer pain (BCP) represents one of the most challenging comorbidities associated with cancer metastasis. Long non-coding RNAs (lncRNAs) have garnered attention as potential therapeutic agents in managing neuropathic pain. However, their role in the regulation of nociceptive information processing remains poorly understood. In this study, we observed a significant down-regulation of the spinal lncRNA ENSRNOG00000051325 (lncRNA51325) in a rat model of bone cancer pain. Our study sought to elucidate the potential involvement of lncRNA51325 in the development of BCP by modulating the expression of molecules associated with pain modulation.
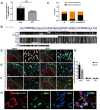
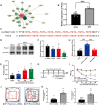
Methods: We established the BCP model by injecting Walker 256 cells into the tibial plateau of rats. We conducted tests on the pain behaviors and anxiety-like responses of rats through von-Frey test, Gait analysis, and Open Field Test. Spinal lumbar expansion was harvested for molecular biology experiments to explore the relationship between lncRNA51325 and Pumilio RNA binding family member 2 (Pum2).
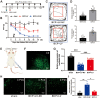
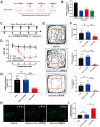
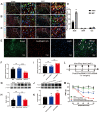
Results: Notably, the overexpression of lncRNA51325 effectively attenuated mechanical allodynia in rats afflicted with BCP, whereas the knockdown of lncRNA51325 induced pain behaviors and anxiety-like responses in naïve rats. Additionally, we observed a time-dependent increase in the expression of Pum2 in BCP-afflicted rats, and intrathecal injection of Pum2-siRNA alleviated hyperalgesia. Furthermore, our investigations revealed that lncRNA51325 exerts a negative modulatory effect on Pum2 expression. The overexpression of lncRNA51325 significantly suppressed Pum2 expression in BCP rats, while the knockdown of lncRNA51325 led to elevated Pum2 protein levels in the spinal cord of naïve rats. Subsequent treatment with Pum2-siRNA mitigated the downregulation of lncRNA51325-induced mechanical allodynia in naïve rats.
Conclusion: Our findings indicate that lncRNA51325 plays a role in regulating bone cancer pain by inhibiting Pum2 expression, offering a promising avenue for novel treatments targeting nociceptive hypersensitivity induced by bone metastatic cancer.
Keywords: Pum2; bone cancer pain; long non-coding RNA; spinal cord.
© 2024 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Analgesic effect of electroacupuncture on bone cancer pain in rat model: the role of peripheral P2X3 receptor.Purinergic Signal. 2023 Mar;19(1):13-27. doi: 10.1007/s11302-022-09861-7. Epub 2022 Apr 28. Purinergic Signal. 2023. PMID: 35478452 Free PMC article.
-
Upregulation of LncRNA71132 in the spinal cord regulates hypersensitivity in a rat model of bone cancer pain.Pain. 2023 Jan 1;164(1):180-196. doi: 10.1097/j.pain.0000000000002678. Epub 2022 May 11. Pain. 2023. PMID: 35543644
-
miR-155-5p in the spinal cord regulates hypersensitivity in a rat model of bone cancer pain.Mol Pain. 2022 Apr;18:17448069221127811. doi: 10.1177/17448069221127811. Mol Pain. 2022. PMID: 36069070 Free PMC article.
-
Suppression of HDAC2 in Spinal Cord Alleviates Mechanical Hyperalgesia and Restores KCC2 Expression in a Rat Model of Bone Cancer Pain.Neuroscience. 2018 May 1;377:138-149. doi: 10.1016/j.neuroscience.2018.02.026. Epub 2018 Feb 23. Neuroscience. 2018. PMID: 29482000
-
Minocycline attenuates bone cancer pain in rats by inhibiting NF-κB in spinal astrocytes.Acta Pharmacol Sin. 2016 Jun;37(6):753-62. doi: 10.1038/aps.2016.1. Epub 2016 May 9. Acta Pharmacol Sin. 2016. PMID: 27157092 Free PMC article.
Cited by
-
Role of ncRNAs in the Development of Chronic Pain.Noncoding RNA. 2025 Jul 3;11(4):51. doi: 10.3390/ncrna11040051. Noncoding RNA. 2025. PMID: 40700093 Free PMC article. Review.
-
Analysis of Ionomic Profiles of Spinal Cords in a Rat Model with Bone Cancer Pain.J Pain Res. 2024 Apr 23;17:1531-1545. doi: 10.2147/JPR.S447282. eCollection 2024. J Pain Res. 2024. PMID: 38682106 Free PMC article.
References
LinkOut - more resources
Full Text Sources

