Berberine mediated fluorescent gold nanoclusters in biomimetic erythrocyte ghosts as a nanocarrier for enhanced photodynamic treatment
- PMID: 38249664
- PMCID: PMC10798219
- DOI: 10.1039/d3ra08299g
Berberine mediated fluorescent gold nanoclusters in biomimetic erythrocyte ghosts as a nanocarrier for enhanced photodynamic treatment
Abstract
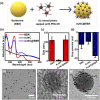
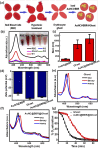
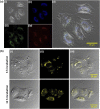
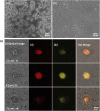
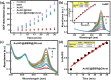
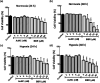
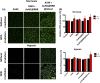
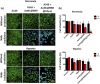
Photodynamic therapy (PDT) is a well-established cancer treatment method that employs light to generate reactive oxygen species (ROS) causing oxidative damage to cancer cells. Nevertheless, PDT encounters challenges due to its oxygen-dependent nature, which makes it less effective in hypoxic tumor environments. To address this issue, we have developed a novel nanocomposite known as AuNC@BBR@Ghost. This nanocomposite combines the advantageous features of erythrocyte ghost membranes, the photoresponsive properties of gold nanoclusters (AuNC) and the anticancer characteristics of Berberine (BBR) for cancer treatment. Our synthesized AuNC efficiently produce ROS, with a 25% increase in efficiency when exposed to near-infrared (NIR) irradiation. By harnessing the oxygen-carrying capacity of erythrocyte ghost cells, AuNC@BBR@Ghost demonstrates a significant improvement in ROS generation, achieving an 80% efficiency. Furthermore, the AuNC exhibit tunable emission wavelengths due to their excellent fluorescent properties. In normoxic conditions, treatment of A549 lung carcinoma cells with AuNC@BBR@Ghost followed by exposure to 808 nm NIR irradiation results in a notable increase in intracellular ROS levels, accelerating cell death. In hypoxic conditions, when A549 cells were treated with AuNC@BBR@Ghost, the erythrocyte ghost acted as an oxygen supplement due to the residual hemoglobin, alleviating hypoxia and enhancing the nanocomposite's sensitivity to PDT treatment. Thus, the AuNC@BBR@Ghost nanocomposite achieves an improved effect by combining the advantageous properties of its individual components, resulting in enhanced ROS generation and adaptability to hypoxic conditions. This innovative approach successfully overcomes PDT's limitations, making AuNC@BBR@Ghost a promising nanotheranostic agent with significant potential for advanced cancer therapy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare there are no conflicts of interest to declare.
Figures
Similar articles
-
Low-dose X-ray radiodynamic therapy solely based on gold nanoclusters for efficient treatment of deep hypoxic solid tumors combined with enhanced antitumor immune response.Theranostics. 2023 Jan 22;13(3):1042-1058. doi: 10.7150/thno.78649. eCollection 2023. Theranostics. 2023. PMID: 36793856 Free PMC article.
-
Berberine-photodynamic induced apoptosis by activating endoplasmic reticulum stress-autophagy pathway involving CHOP in human malignant melanoma cells.Biochem Biophys Res Commun. 2021 May 7;552:183-190. doi: 10.1016/j.bbrc.2021.02.147. Epub 2021 Mar 19. Biochem Biophys Res Commun. 2021. PMID: 33751936
-
A new NIR-triggered doxorubicin and photosensitizer indocyanine green co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy.Acta Biomater. 2017 Sep 1;59:170-180. doi: 10.1016/j.actbio.2017.06.026. Epub 2017 Jun 17. Acta Biomater. 2017. PMID: 28629893
-
Research progress of berberine mediated photodynamic therapy.Oncol Lett. 2021 May;21(5):359. doi: 10.3892/ol.2021.12620. Epub 2021 Mar 8. Oncol Lett. 2021. PMID: 33747216 Free PMC article. Review.
-
Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy.Int J Mol Sci. 2023 Nov 29;24(23):16890. doi: 10.3390/ijms242316890. Int J Mol Sci. 2023. PMID: 38069213 Free PMC article. Review.
Cited by
-
Integrating Chinese medicine into mainstream cancer therapies: a promising future.Front Oncol. 2024 Jun 18;14:1412370. doi: 10.3389/fonc.2024.1412370. eCollection 2024. Front Oncol. 2024. PMID: 38957318 Free PMC article. Review.
-
Red blood cells-derived components as biomimetic functional materials: Matching versatile delivery strategies based on structure and function.Bioact Mater. 2025 Feb 13;47:481-501. doi: 10.1016/j.bioactmat.2025.01.021. eCollection 2025 May. Bioact Mater. 2025. PMID: 40034412 Free PMC article. Review.
-
Transforming Cancer Treatment with Nanotechnology: The Role of Berberine as a Star Natural Compound.Int J Nanomedicine. 2024 Aug 22;19:8621-8640. doi: 10.2147/IJN.S469350. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39188860 Free PMC article. Review.
References
-
- Zhang L. Wang E. Nano Today. 2014;9:132–157.
-
- Londoño-Larrea P. Vanegas J. P. Cuaran-Acosta D. Zaballos-García E. Pérez-Prieto J. Chem.—Eur. J. 2017;23:8137–8141. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

