Dissecting the genetic variations associated with response to first-line chemotherapy in patients with small cell lung cancer: a retrospective cohort study
- PMID: 38249933
- PMCID: PMC10797352
- DOI: 10.21037/jtd-23-1772
Dissecting the genetic variations associated with response to first-line chemotherapy in patients with small cell lung cancer: a retrospective cohort study
Abstract
Background: Chemotherapy has been the standard treatment for small-cell lung cancer (SCLC) for decades. Nonetheless, patients are usually responsive to initial chemotherapy but quickly suffer from relapse, resulting in a poor long-term outcome. Treating advances that greatly ameliorate survival outcomes are historically finite, and credible biomarkers for therapeutic evaluation are deficient. As the genetic biology emerges, investigating biomarkers to optimize individualized treatment for SCLC is necessary.
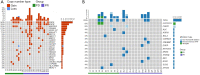
Methods: Based on following inclusion criteria: (I) patients diagnosed as SCLC by pathology; (II) patients treated with first-line etoposide/cisplatin (EP) chemotherapy; (III) patients who received long-term follow-up and signed informed consent, a total of 24 SCLC patients receiving first-line standard chemotherapy were divided into progressive disease (PD) and partial response (PR) groups. They were regularly followed every 3 months with computed tomography (CT) scan until recurrences determined by CT scan results. Next-generation sequencing (NGS) with a panel of 1,406 cancer-related genes was conducted on the tumor tissue-derived DNA of patients to compare genetic variations, including deletions (indels), single nucleotide variations (SNVs), copy number variations (CNVs), and copy number instability (CNI) between the two groups.
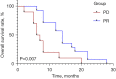
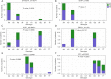
Results: For the clinical characteristics of enrolled SCLC patients, except for significant differences in sex, age, clinical stage, and limited or extensive stage, PD patients showed distinctly shorter overall survival than those with PR (6.5 vs. 14.0 months, respectively, P=0.007). Genetic variations analysis discovered several common genes with CNV mutations between the PR and PD groups, and increased epidermal growth factor receptor (EGFR) gene copy numbers gain was found in PR groups in comparing with PD patients (P=0.006). However, no significant differences in terms of SNVs, indels, genotypes associated with first-line chemotherapy, CNI of tumor tissue-derived DNA, and tumor mutational burden of tumor tissues were observed between two groups. Additionally, the relationship between EGFR gene mutation and clinicopathological features of SCLC indicated that EGFR gene mutation may be an independent indicator for SCLC patients.
Conclusions: Increased EGFR gene CNVs may be an independent indicator influencing the survival time and PR in SCLC patients receiving standard first-line chemotherapy.
Keywords: Epidermal growth factor receptor (EGFR); first-line chemotherapy; genetic variation; small cell lung cancer (SCLC); somatic mutation.
2023 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1772/coif). H.Z. is from Genecast Biotechnology Co. Ltd., Wuxi, Jiangsu, China. J.Z. is from HaploX Biotechnology, Shenzhen, Guangdong, China. The other authors have no conflicts of interest to declare.
Figures

Similar articles
-
Epidermal Growth Factor Receptor Mutation (EGFR) Testing for Prediction of Response to EGFR-Targeting Tyrosine Kinase Inhibitor (TKI) Drugs in Patients with Advanced Non-Small-Cell Lung Cancer: An Evidence-Based Analysis.Ont Health Technol Assess Ser. 2010;10(24):1-48. Epub 2010 Dec 1. Ont Health Technol Assess Ser. 2010. PMID: 23074402 Free PMC article.
-
Molecular evolutionary process of advanced gastric cancer during sequential chemotherapy detected by circulating tumor DNA.J Transl Med. 2022 Aug 12;20(1):365. doi: 10.1186/s12967-022-03567-5. J Transl Med. 2022. PMID: 35962408 Free PMC article.
-
Whole exome sequencing (WES) analysis of transformed small cell lung cancer (SCLC) from lung adenocarcinoma (LUAD).Transl Lung Cancer Res. 2020 Dec;9(6):2428-2439. doi: 10.21037/tlcr-20-1278. Transl Lung Cancer Res. 2020. PMID: 33489804 Free PMC article.
-
Prolonging Survival: The Role of Immune Checkpoint Inhibitors in the Treatment of Extensive-Stage Small Cell Lung Cancer.Oncologist. 2020 Nov;25(11):981-992. doi: 10.1634/theoncologist.2020-0193. Epub 2020 Sep 23. Oncologist. 2020. PMID: 32860288 Free PMC article. Review.
-
Durvalumab: A Review in Extensive-Stage SCLC.Target Oncol. 2021 Nov;16(6):857-864. doi: 10.1007/s11523-021-00843-0. Epub 2021 Nov 3. Target Oncol. 2021. PMID: 34731446 Free PMC article. Review.
Cited by
-
Analysis of exportins expression unveils their prognostic significance in colon adenocarcinoma: insights from public databases.Discov Oncol. 2025 Jan 8;16(1):21. doi: 10.1007/s12672-025-01748-4. Discov Oncol. 2025. PMID: 39776001 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
