The role of the Notch signalling pathway in the pathogenesis of ulcerative colitis: from the perspective of intestinal mucosal barrier
- PMID: 38249980
- PMCID: PMC10796567
- DOI: 10.3389/fmed.2023.1333531
The role of the Notch signalling pathway in the pathogenesis of ulcerative colitis: from the perspective of intestinal mucosal barrier
Abstract
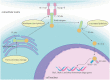
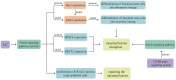
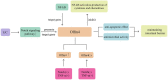
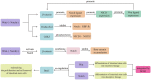
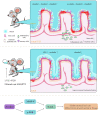
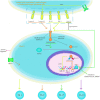
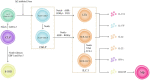
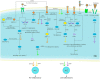
Ulcerative colitis is a common digestive disorder worldwide, with increasing incidence in recent years. It is an urgent problem to be solved, as it seriously affects and threatens the health and life of the global population. Studies have shown that dysfunction of the intestinal mucosal barrier is a critical pathogenic factor and molecular basis of ulcerative colitis, and some scholars have described it as a "barrier organ disease." While the Notch signalling pathway affects a series of cellular processes, including proliferation, differentiation, development, migration, and apoptosis. Therefore, it can regulate intestinal stem cells, CD4+ T cells, innate lymphoid cells, macrophages, and intestinal microbiota and intervene in the chemical, physical, immune, and biological mucosal barriers in cases of ulcerative colitis. The Notch signalling pathway associated with the pathogenesis of ulcerative colitis has distinct characteristics, with good regulatory effects on the mucosal barrier. However, research on ulcerative colitis has mainly focused on immune regulation, anti-inflammatory activity, and antioxidant stress; therefore, the study of the Notch signalling pathway suggests the possibility of understanding the pathogenesis of ulcerative colitis from another perspective. In this article we explore the role and mechanism of the Notch signalling pathway in the pathogenesis of ulcerative colitis from the perspective of the intestinal mucosal barrier to provide new targets and theoretical support for further research on the pathogenesis and clinical treatment of ulcerative colitis.
Keywords: Notch; mucosal barrier; review; signalling pathway; ulcerative colitis.
Copyright © 2024 Ning, Liu, Tan, Yi and Lin.
Conflict of interest statement
HN received the grant from Hunan Province College Students’ Innovation and Entrepreneurship Training Program. JL received the grant from the National Natural Science Foundation of China and the High-Level Innovative Talents Project of Guizhou Province. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
[Research progress of traditional Chinese medicine intervention for ulcerative colitis based on Notch1 signaling pathway].Zhongguo Zhong Yao Za Zhi. 2024 Apr;49(7):1741-1748. doi: 10.19540/j.cnki.cjcmm.20231127.405. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 38812186 Review. Chinese.
-
Repairing the intestinal mucosal barrier of traditional Chinese medicine for ulcerative colitis: a review.Front Pharmacol. 2023 Oct 24;14:1273407. doi: 10.3389/fphar.2023.1273407. eCollection 2023. Front Pharmacol. 2023. PMID: 37942490 Free PMC article. Review.
-
Traditional Chinese Medicine: A promising strategy to regulate the imbalance of bacterial flora, impaired intestinal barrier and immune function attributed to ulcerative colitis through intestinal microecology.J Ethnopharmacol. 2024 Jan 10;318(Pt A):116879. doi: 10.1016/j.jep.2023.116879. Epub 2023 Jul 5. J Ethnopharmacol. 2024. PMID: 37419224 Review.
-
Angelica oil restores the intestinal barrier function by suppressing S100A8/A9 signalling in mice with ulcerative colitis.Phytomedicine. 2023 Jan;108:154490. doi: 10.1016/j.phymed.2022.154490. Epub 2022 Oct 15. Phytomedicine. 2023. PMID: 36332386
-
High-fat diet induces intestinal mucosal barrier dysfunction in ulcerative colitis: emerging mechanisms and dietary intervention perspective.Am J Transl Res. 2023 Feb 15;15(2):653-677. eCollection 2023. Am J Transl Res. 2023. PMID: 36915785 Free PMC article. Review.
Cited by
-
Unveiling the multifaceted roles of anthocyanins: a review of their bioavailability, impacts on gut and system health, and industrial implications.Curr Res Food Sci. 2025 Jul 9;11:101137. doi: 10.1016/j.crfs.2025.101137. eCollection 2025. Curr Res Food Sci. 2025. PMID: 40703150 Free PMC article. Review.
-
Exploring Protein Functions of Gut Bacteriome and Mycobiome in Thai Infants Associated with Atopic Dermatitis Through Metaproteomic and Host Interaction Analysis.Int J Mol Sci. 2024 Dec 18;25(24):13533. doi: 10.3390/ijms252413533. Int J Mol Sci. 2024. PMID: 39769296 Free PMC article.
-
Oxyberberine alleviates lipopolysaccharide-induced intestinal barrier disruption and inflammation in human colonic Caco-2 cells in vitro.Front Pharmacol. 2025 Jan 7;15:1496874. doi: 10.3389/fphar.2024.1496874. eCollection 2024. Front Pharmacol. 2025. PMID: 39840109 Free PMC article.
-
Evaluation of Lacticaseibacillus casei 39 Paraprobiotic in Modulating Inflammatory and Oxidative Pathways in Acetic Acid Induced Ulcerative Colitis.Food Sci Nutr. 2025 Jul 18;13(7):e70476. doi: 10.1002/fsn3.70476. eCollection 2025 Jul. Food Sci Nutr. 2025. PMID: 40688608 Free PMC article.
-
Activation of notch signaling pathway is a potential mechanism for mucin2 reduction and intestinal mucosal barrier dysfunction in high-altitude hypoxia.Sci Rep. 2025 Apr 9;15(1):12154. doi: 10.1038/s41598-025-96176-3. Sci Rep. 2025. PMID: 40204779 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

