Prevention of exacerbation in patients with moderate-to-very severe COPD with the intent to modulate respiratory microbiome: a pilot prospective, multi-center, randomized controlled trial
- PMID: 38249987
- PMCID: PMC10797043
- DOI: 10.3389/fmed.2023.1265544
Prevention of exacerbation in patients with moderate-to-very severe COPD with the intent to modulate respiratory microbiome: a pilot prospective, multi-center, randomized controlled trial
Abstract
Introduction: Considering the role of bacteria in the onset of acute exacerbation of COPD (AECOPD), we hypothesized that the use of influenza-Streptococcus pneumoniae vaccination, oral probiotics or inhaled amikacin could prevent AECOPD.
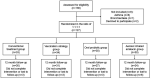
Methods: In this pilot prospective, muti-central, randomized trial, moderate-to-very severe COPD subjects with a history of moderate-to-severe exacerbations in the previous year were enrolled and assigned in a ratio of 1:1:1:1 into 4 groups. All participants were managed based on the conventional treatment recommended by GOLD 2019 report for 3 months, with three groups receiving additional treatment of inhaled amikacin (0.4 g twice daily, 5-7 days monthly for 3 months), oral probiotic Lactobacillus rhamnosus GG (1 tablet daily for 3 months), or influenza-S. pneumoniae vaccination. The primary endpoint was time to the next onset of moderate-to-severe AECOPD from enrollment. Secondary endpoints included CAT score, mMRC score, adverse events, and survival in 12 months.
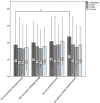
Results: Among all 112 analyzed subjects (101 males, 96 smokers or ex-smokers, mean ± SD age 67.19 ± 7.39 years, FEV1 41.06 ± 16.09% predicted), those who were given dual vaccination (239.7 vs. 198.2 days, p = 0.044, 95%CI [0.85, 82.13]) and oral probiotics (248.8 vs. 198.2 days, p = 0.017, 95%CI [7.49, 93.59]) had significantly delayed onset of next moderate-to-severe AECOPD than those received conventional treatment only. For subjects with high symptom burden, the exacerbations were significantly delayed in inhaled amikacin group as compared to the conventional treatment group (237.3 vs. 179.1 days, p = 0.009, 95%CI [12.40,104.04]). The three interventions seemed to be safe and well tolerated for patient with stable COPD.
Conclusion: The influenza-S. pneumoniae vaccine and long-term oral probiotic LGG can significantly delay the next moderate-to-severe AECOPD. Periodically amikacin inhalation seems to work in symptomatic patients. The findings in the current study warrants validation in future studies with microbiome investigation.Clinical trial registration:https://clinicaltrials.gov/, identifier NCT03449459.
Keywords: amikacin; chronic obstructive pulmonary disease; inhaled antibiotics; probiotic; vaccination.
Copyright © 2024 Hua, Yang, Cheng, Han, Li, Dai, He, Wu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Prevention of Acute Exacerbation in Subjects with Moderate-to-very Severe COPD by Modulating Lower Respiratory Microbiome: Protocol of a Prospective, Multicenter, Randomized Controlled Trial.Int J Chron Obstruct Pulmon Dis. 2020 Nov 17;15:2985-2990. doi: 10.2147/COPD.S274005. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 33235447 Free PMC article.
-
Non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine for the prevention of exacerbations in chronic obstructive pulmonary disease: a multicentre, randomised, placebo-controlled, observer-blinded, proof-of-concept, phase 2b trial.Lancet Respir Med. 2022 May;10(5):435-446. doi: 10.1016/S2213-2600(21)00502-6. Epub 2022 Jan 10. Lancet Respir Med. 2022. PMID: 35026180 Clinical Trial.
-
Effect of sequential treatment with syndrome differentiation on acute exacerbation of chronic obstructive pulmonary disease and "AECOPD Risk-Window": study protocol for a randomized placebo-controlled trial.Trials. 2012 Apr 20;13:40. doi: 10.1186/1745-6215-13-40. Trials. 2012. PMID: 22520863 Free PMC article. Clinical Trial.
-
Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2019 Mar 6;3(3):CD012930. doi: 10.1002/14651858.CD012930.pub2. Cochrane Database Syst Rev. 2019. PMID: 30839102 Free PMC article.
-
Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD.Treat Respir Med. 2004;3(4):247-68. doi: 10.2165/00151829-200403040-00005. Treat Respir Med. 2004. PMID: 15350163 Review.
Cited by
-
Gut Microbiota Modulation in the Management of Chronic Obstructive Pulmonary Disease: A Literature Review.Cureus. 2024 Aug 14;16(8):e66875. doi: 10.7759/cureus.66875. eCollection 2024 Aug. Cureus. 2024. PMID: 39280360 Free PMC article. Review.
-
The Influence of the Gut Microbiota on Host Health: A Focus on the Gut-Lung Axis and Therapeutic Approaches.Life (Basel). 2024 Oct 9;14(10):1279. doi: 10.3390/life14101279. Life (Basel). 2024. PMID: 39459579 Free PMC article. Review.
-
The role and mechanism of gut-lung axis mediated bidirectional communication in the occurrence and development of chronic obstructive pulmonary disease.Gut Microbes. 2024 Jan-Dec;16(1):2414805. doi: 10.1080/19490976.2024.2414805. Epub 2024 Oct 24. Gut Microbes. 2024. PMID: 39446051 Free PMC article. Review.
References
-
- Institute for Health Metrics and Evaluation (IHME) . GBD Compare Data Visualization. IHME, University of Washington. (2019). Available at: https://vizhub.healthdata.org/gbd-compare/ (Accessed September 23, 2022).
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

