Morphine- and foot shock-responsive neuronal ensembles in the VTA possess different connectivity and biased GPCR signaling pathway
- PMID: 38250036
- PMCID: PMC10797299
- DOI: 10.7150/thno.90792
Morphine- and foot shock-responsive neuronal ensembles in the VTA possess different connectivity and biased GPCR signaling pathway
Abstract
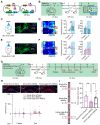
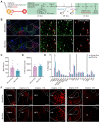
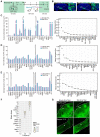
Background: Neurons in the ventral tegmental area (VTA) are sensitive to stress and their maladaptation have been implicated in the psychiatric disorders such as anxiety and addiction, etc. The cellular properties of the VTA neurons in response to different stressors related to different emotional processing remain to be investigated. Methods: By combining immediate early gene (IEG)-dependent labeling, rabies virus tracing, ensemble-specific transcriptomic analysis and fiber photometry recording in the VTA of male mice, the spatial distribution, brain-wide connectivity and cellular signaling pathways in the VTA neuronal ensembles in response to morphine (Mor-Ens) or foot shock (Shock-Ens) stimuli were investigated. Results: Optogenetic activation of the Mor-Ens drove approach behavior, whereas chemogenetic activation of the Shock-Ens increased the anxiety level in mice. Mor-Ens were clustered and enriched in the ventral VTA, contained a higher proportion of dopaminergic neurons, received more inputs from the dorsal medial striatum and the medial hypothalamic zone, and exhibited greater axonal arborization in the zona incerta and ventral pallidum. Whereas Shock-Ens were more dispersed, contained a higher proportion of GABAergic neurons, and received more inputs from the ventral pallidum and the lateral hypothalamic area. The downstream targets of the G protein and β-arrestin pathways, PLCβ3 and phosphorylated AKT1Thr308, were relatively enriched in the Mor-Ens and Shock-Ens, respectively. Cariprazine, the G-protein-biased agonist for the dopamine D2 receptor, increased the response of Mor-Ens to sucrose water and decreased the anxiety-like behavior during morphine withdrawal, whereas the β-arrestin-biased agonist UNC9994 decreased the response of Shock-Ens to tail suspension. Conclusions: Taken together, these findings reveal the heterogeneous connectivity and signaling pathways of the VTA neurons in response to morphine and foot shock, providing new insights for development of specific interventions for psychiatric disorders caused by various stressors associated with different VTA neuronal functions.
Keywords: Ensemble; Foot shock; GPCR; Morphine; Ventral tegmental area.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Craske MG, Stein MB. Anxiety. Lancet. 2016;388:3048–59. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

