Construction of tumor organoids and their application to cancer research and therapy
- PMID: 38250041
- PMCID: PMC10797287
- DOI: 10.7150/thno.91362
Construction of tumor organoids and their application to cancer research and therapy
Abstract
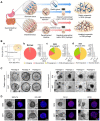
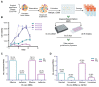
Cancer remains a severe public health burden worldwide. One of the challenges hampering effective cancer therapy is that the existing cancer models hardly recapitulate the tumor microenvironment of human patients. Over the past decade, tumor organoids have emerged as an in vitro 3D tumor model to mimic the pathophysiological characteristics of parental tumors. Various techniques have been developed to construct tumor organoids, such as matrix-based methods, hanging drop, spinner or rotating flask, nonadhesive surface, organ-on-a-chip, 3D bioprinting, and genetic engineering. This review elaborated on cell components and fabrication methods for establishing tumor organoid models. Furthermore, we discussed the application of tumor organoids to cancer modeling, basic cancer research, and anticancer therapy. Finally, we discussed current limitations and future directions in employing tumor organoids for more extensive applications.
Keywords: antitumor therapy; basic cancer research; cellular components; culture methods; tumor organoids.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Converging bioprinting and organoids to better recapitulate the tumor microenvironment.Trends Biotechnol. 2024 May;42(5):648-663. doi: 10.1016/j.tibtech.2023.11.006. Epub 2023 Dec 9. Trends Biotechnol. 2024. PMID: 38071145 Review.
-
Reconstructing the tumor architecture into organoids.Adv Drug Deliv Rev. 2021 Sep;176:113839. doi: 10.1016/j.addr.2021.113839. Epub 2021 Jun 19. Adv Drug Deliv Rev. 2021. PMID: 34153370 Free PMC article. Review.
-
Organoids for Cancer Research: Advances and Challenges.Adv Biol (Weinh). 2024 Sep;8(9):e2400056. doi: 10.1002/adbi.202400056. Epub 2024 Jul 8. Adv Biol (Weinh). 2024. PMID: 38977414 Review.
-
Tumor organoids: synergistic applications, current challenges, and future prospects in cancer therapy.Cancer Commun (Lond). 2021 Dec;41(12):1331-1353. doi: 10.1002/cac2.12224. Epub 2021 Oct 29. Cancer Commun (Lond). 2021. PMID: 34713636 Free PMC article. Review.
-
Challenges in Bio-fabrication of Organoid Cultures.Adv Exp Med Biol. 2018;1107:53-71. doi: 10.1007/5584_2018_216. Adv Exp Med Biol. 2018. PMID: 29855825 Review.
Cited by
-
3D tumor cultures for drug resistance and screening development in clinical applications.Mol Cancer. 2025 Mar 21;24(1):93. doi: 10.1186/s12943-025-02281-2. Mol Cancer. 2025. PMID: 40119343 Free PMC article. Review.
-
Applications and perspectives of tumor organoids in radiobiology (Review).Oncol Rep. 2024 Aug;52(2):100. doi: 10.3892/or.2024.8759. Epub 2024 Jun 21. Oncol Rep. 2024. PMID: 38904192 Free PMC article. Review.
-
The Role of Cancer Organoids in Ferroptosis, Pyroptosis, and Necroptosis: Functions and Clinical Implications.Biomolecules. 2025 May 2;15(5):659. doi: 10.3390/biom15050659. Biomolecules. 2025. PMID: 40427552 Free PMC article. Review.
-
Cancer patient-derived organoids: Novel models for the study of natural products.Int J Biol Sci. 2025 Jul 11;21(10):4485-4503. doi: 10.7150/ijbs.114373. eCollection 2025. Int J Biol Sci. 2025. PMID: 40765827 Free PMC article. Review.
-
Reversible chemoresistance of pancreatic cancer grown as spheroids.J Chemother. 2024 Sep 16:1-15. doi: 10.1080/1120009X.2024.2402177. Online ahead of print. J Chemother. 2024. PMID: 39282901
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–45. - PubMed
-
- McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov. 2013;12:217–28. - PubMed
-
- Zeigerer A, Wuttke A, Marsico G, Seifert S, Kalaidzidis Y, Zerial M. Functional properties of hepatocytes in vitro are correlated with cell polarity maintenance. Exp Cell Res. 2017;350:242–52. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

