Humoral and cell-mediated immune responses in HIV-vertically infected young patients after three doses of the BNT162b2 mRNA SARS-CoV-2 vaccine
- PMID: 38250079
- PMCID: PMC10797701
- DOI: 10.3389/fimmu.2023.1301766
Humoral and cell-mediated immune responses in HIV-vertically infected young patients after three doses of the BNT162b2 mRNA SARS-CoV-2 vaccine
Abstract
Background: Data on the efficacy of three SARS-CoV-2 mRNA BNT162b2 vaccine doses and the role of previous SARS-CoV-2-infection in enhancing vaccine immunogenicity in HIV-vertically-infected people living with HIV (PLWH) are limited, as is the duration of vaccine-induced responses.
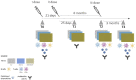
Methods: SARS-CoV-2 plasma neutralizing activity (NA) against the European (B.1), Delta (B.1.617.2) and Omicron (B.1.1.529) variants and cell-mediated immunity (CMI) were analyzed in 29 ART-treated young PLWH (mean age 27.9 years) and 30 healthy controls (HC) who received three BNT162b2 vaccine doses. Individuals were stratified based on the presence/absence of previous SARS-CoV-2 infection (infected and vaccinated -SIV-; uninfected and vaccinated -SV-). Analyses were performed before vaccination (T0), 25 days from the second dose (T1), the day the third dose was administered (T2), and 3 months after the third dose (T3).
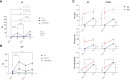
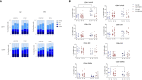
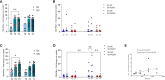
Results: In PLWH: i) NA against all variants was higher in SIV compared to SV at T2 and was increased at T3; ii) switched-memory plasmablasts were augmented in SIV alone at T2 and T3; iii) a SARS-CoV-2 specific T cell memory was generated; iv) IFN-γ-secreting CD4+ and CD8+ T lymphocytes were boosted at T3 mainly in SV. CMI magnitude was reduced in PLWH compared to HC. Notably, after the third dose of vaccine viremia was unmodified, but CD4 T cell counts were reduced>20% in 3/29 PHLW.
Conclusion: A third dose of BNT162b2 vaccine induces strong humoral and CMI responses in young ART-treated PLWH independently from a previous SARS-CoV-2 natural infection. The lower magnitude of CMI responses should be considered when planning mRNA vaccine booster doses in PLWH.
Keywords: COVID-19 SIV individuals; PLWH; SARS-CoV-2 vaccine efficacy; immune response; immunocompromised subjects.
Copyright © 2024 Vanetti, Stracuzzi, Crivellaro, Ciciliano, Garziano, Fenizia, Biasin, Rubinacci, Amendola, Tanzi, Zuccotti, Clerici, Giacomet and Trabattoni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet (2021) 397:1819–29. doi: 10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
-
- Muhsen K, Maimon N, Mizrahi AY, Varticovschi B, Bodenheimer O, Cohen D, et al. Association of BNT162b2 vaccine third dose receipt with incidence of SARS-coV-2 infection, COVID-19–related hospitalization, and death among residents of long-term care facilities, august to october 2021. JAMA Netw Open (2022) 5:e2219940. doi: 10.1001/jamanetworkopen.2022.19940 - DOI - PMC - PubMed
-
- Morrocchi E, Pighi C, Pascucci GR, Cotugno N, Medri C, Amodio D, et al. Perinatally human immunodeficiency virus–infected adolescents and young adults demonstrate distinct BNT162b2 messenger RNA coronavirus disease 2019 vaccine immunogenicity. Clin Infect Dis (2022) 75:S51–60. doi: 10.1093/cid/ciac408 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

