Reduced monocyte proportions and responsiveness in convalescent COVID-19 patients
- PMID: 38250080
- PMCID: PMC10797708
- DOI: 10.3389/fimmu.2023.1329026
Reduced monocyte proportions and responsiveness in convalescent COVID-19 patients
Abstract
Introduction: The clinical manifestations of acute severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) suggest a dysregulation of the host immune response that leads to inflammation, thrombosis, and organ dysfunction. It is less clear whether these dysregulated processes persist during the convalescent phase of disease or during long COVID. We sought to examine the effects of SARS-CoV-2 infection on the proportions of classical, intermediate, and nonclassical monocytes, their activation status, and their functional properties in convalescent COVID-19 patients.
Methods: Peripheral blood mononuclear cells (PBMCs) from convalescent COVID-19 patients and uninfected controls were analyzed by multiparameter flow cytometry to determine relative percentages of total monocytes and monocyte subsets. The expression of activation markers and proinflammatory cytokines in response to LPS treatment were measured by flow cytometry and ELISA, respectively.
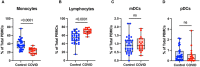
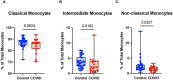
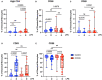
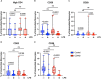
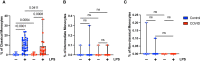
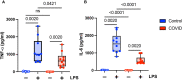
Results: We found that the percentage of total monocytes was decreased in convalescent COVID-19 patients compared to uninfected controls. This was due to decreased intermediate and non-classical monocytes. Classical monocytes from convalescent COVID-19 patients demonstrated a decrease in activation markers, such as CD56, in response to stimulation with bacterial lipopolysaccharide (LPS). In addition, classical monocytes from convalescent COVID-19 patients showed decreased expression of CD142 (tissue factor), which can initiate the extrinsic coagulation cascade, in response to LPS stimulation. Finally, we found that monocytes from convalescent COVID-19 patients produced less TNF-α and IL-6 in response to LPS stimulation, than those from uninfected controls.
Conclusion: SARS-CoV-2 infection exhibits a clear effect on the relative proportions of monocyte subsets, the activation status of classical monocytes, and proinflammatory cytokine production that persists during the convalescent phase of disease.
Keywords: 70; COVID-19; IL-6 69; SARS-CoV-2; TNF-α; monocytes; tissue factor.
Copyright © 2024 Ravkov, Williams, Elgort, Barker, Planelles, Spivak, Delgado, Lin and Hanley.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Update of
-
Reduced Monocyte Proportions and Responsiveness in Convalescent COVID-19 Patients.bioRxiv [Preprint]. 2023 Oct 26:2023.10.25.563806. doi: 10.1101/2023.10.25.563806. bioRxiv. 2023. Update in: Front Immunol. 2024 Jan 04;14:1329026. doi: 10.3389/fimmu.2023.1329026. PMID: 37961575 Free PMC article. Updated. Preprint.
Comment on
-
Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection.Nat Immunol. 2022 Feb;23(2):210-216. doi: 10.1038/s41590-021-01113-x. Epub 2022 Jan 13. Nat Immunol. 2022. PMID: 35027728
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

