Corticosteroids in Sepsis and Septic Shock: A Systematic Review, Pairwise, and Dose-Response Meta-Analysis
- PMID: 38250247
- PMCID: PMC10798738
- DOI: 10.1097/CCE.0000000000001000
Corticosteroids in Sepsis and Septic Shock: A Systematic Review, Pairwise, and Dose-Response Meta-Analysis
Abstract
Objectives: To perform a systematic review and meta-analysis to assess the efficacy and safety of corticosteroids in patients with sepsis.
Data sources: We searched PubMed, Embase, and the Cochrane Library, up to January 10, 2023.
Study selection: We included randomized controlled trials (RCTs) comparing corticosteroids with placebo or standard care with sepsis.
Data extraction: The critical outcomes of interest included mortality, shock reversal, length of stay in the ICU, and adverse events.
Data analysis: We performed both a pairwise and dose-response meta-analysis to evaluate the effect of different corticosteroid doses on outcomes. We used Grading of Recommendations Assessment, Development and Evaluation to assess certainty in pooled estimates.
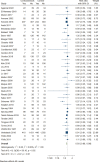
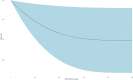
Data synthesis: We included 45 RCTs involving 9563 patients. Corticosteroids probably reduce short-term mortality (risk ratio [RR], 0.93; 95% CI, 0.88-0.99; moderate certainty) and increase shock reversal at 7 days (RR, 1.24; 95% CI, 1.11-1.38; high certainty). Corticosteroids may have no important effect on duration of ICU stay (mean difference, -0.6 fewer days; 95% CI, 1.48 fewer to 0.27 more; low certainty); however, probably increase the risk of hyperglycemia (RR, 1.13; 95% CI, 1.08-1.18; moderate certainty) and hypernatremia (RR, 1.64; 95% CI, 1.32-2.03; moderate certainty) and may increase the risk of neuromuscular weakness (RR, 1.21; 95% CI, 1.01-1.45; low certainty). The dose-response analysis showed a reduction in mortality with corticosteroids with optimal dosing of approximately 260 mg/d of hydrocortisone (RR, 0.90; 95% CI, 0.83-0.98) or equivalent.
Conclusions: We found that corticosteroids may reduce mortality and increase shock reversal but they may also increase the risk of hyperglycemia, hypernatremia, and neuromuscular weakness. The dose-response analysis indicates optimal dosing is around 260 mg/d of hydrocortisone or equivalent.
Keywords: corticosteroids; critical illness; meta-analysis; sepsis; septic shock.
Copyright © 2024 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
The authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources