Immune cells and RBCs derived from human induced pluripotent stem cells: method, progress, prospective challenges
- PMID: 38250324
- PMCID: PMC10796611
- DOI: 10.3389/fcell.2023.1327466
Immune cells and RBCs derived from human induced pluripotent stem cells: method, progress, prospective challenges
Abstract
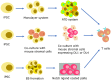
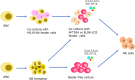
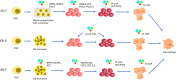
Blood has an important role in the healthcare system, particularly in blood transfusions and immunotherapy. However, the occurrence of outbreaks of infectious diseases worldwide and seasonal fluctuations, blood shortages are becoming a major challenge. Moreover, the narrow specificity of immune cells hinders the widespread application of immune cell therapy. To address this issue, researchers are actively developing strategies for differentiating induced pluripotent stem cells (iPSCs) into blood cells in vitro. The establishment of iPSCs from terminally differentiated cells such as fibroblasts and blood cells is a straightforward process. However, there is need for further refinement of the protocols for differentiating iPSCs into immune cells and red blood cells to ensure their clinical applicability. This review aims to provide a comprehensive overview of the strategies and challenges facing the generation of iPSC-derived immune cells and red blood cells.
Keywords: iPSC (induced pluripotent stem cell); iPSC dereved iNKT cell; iPSC derivation; iPSC derived NK cell; iPSC derived T cell; iPSC derived macrophages cell; red blood cell.
Copyright © 2024 Jiang, Ren, Cheng, Li and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Engineering Induced Pluripotent Stem Cells for Cancer Immunotherapy.Cancers (Basel). 2022 May 1;14(9):2266. doi: 10.3390/cancers14092266. Cancers (Basel). 2022. PMID: 35565395 Free PMC article. Review.
-
Induced Pluripotent Stem Cell (iPSC)-Derived Lymphocytes for Adoptive Cell Immunotherapy: Recent Advances and Challenges.Curr Hematol Malig Rep. 2019 Aug;14(4):261-268. doi: 10.1007/s11899-019-00528-6. Curr Hematol Malig Rep. 2019. PMID: 31243643 Free PMC article. Review.
-
Regeneration of invariant natural killer T (iNKT) cells: application of iPSC technology for iNKT cell-targeted tumor immunotherapy.Inflamm Regen. 2023 May 12;43(1):27. doi: 10.1186/s41232-023-00275-5. Inflamm Regen. 2023. PMID: 37170375 Free PMC article. Review.
-
Adoptive Cell Therapy from the Dish: Potentiating Induced Pluripotent Stem Cells.Transfus Med Hemother. 2024 Aug 26;52(1):27-41. doi: 10.1159/000540473. eCollection 2025 Feb. Transfus Med Hemother. 2024. PMID: 39944411 Free PMC article. Review.
-
Supporting materials: Endothelial cells differentiated from patient dermal fibroblast-derived induced pluripotent stem cells resemble vascular malformations of Port Wine Birthmark.bioRxiv [Preprint]. 2023 Aug 24:2023.07.02.547408. doi: 10.1101/2023.07.02.547408. bioRxiv. 2023. Update in: Br J Dermatol. 2023 Nov 16;189(6):780-783. doi: 10.1093/bjd/ljad330. PMID: 37662218 Free PMC article. Updated. Preprint.
References
Publication types
LinkOut - more resources
Full Text Sources

