Dual Effect of Secondary Solutes on Binding Equilibria: Contributions from Solute-Reactant Interactions and Solute-Water Interactions
- PMID: 38250344
- PMCID: PMC10795149
- DOI: 10.1021/acsomega.3c09329
Dual Effect of Secondary Solutes on Binding Equilibria: Contributions from Solute-Reactant Interactions and Solute-Water Interactions
Abstract
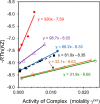
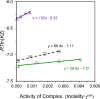
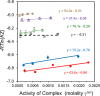
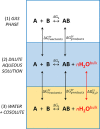
This study examines the role of water in binding equilibria with a special focus on secondary solutes (cosolutes) that influence the equilibrium but are not constituents of the final product. Using a thermodynamic framework that includes an explicit term for the release of water molecules upon binding, this investigation reveals how solutes may alter equilibria by changing the activity of the reactants, reflected in ΔG°(obs), and by changing the chemical potential of the solvent, reflected in ΔGS. The framework is applied to four experimental binding systems that differ in the degree of electrostatic contributions. The model systems include the chelation of Ca2+ by EDTA and three host-guest reactions; the pairings of p-sulfonatocalix[4]arene with tetramethylammonium ion, cucurbit[7]uril with N-acetyl-phenylalanine-amide, and β-cyclodextrin with adamantane carboxylate are tested. Each reaction pair is examined by isothermal titration calorimetry at 25 °C in the presence of a common osmolyte, sucrose, and a common chaotrope, urea. Molar solutions of trehalose and phosphate were also tested with selected models. In general, cosolutes that enhance binding tend to reduce the solvation free energy penalty and cosolutes that weaken binding tend to increase the solvation free energy penalty. Notably, the nonpolar-nonpolar interaction between adamantane carboxylate and β-cyclodextrin is characterized by a ΔGS value near zero. The results with β-cyclodextrin, in particular, prompt further discussions of the hydrophobic effect and the biocompatible properties of trehalose. Other investigators are encouraged to test and refine the approach taken here to further our understanding of solvent effects on molecular recognition.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Eggers D. K.; Brewer A.; Cacatian K. J.; Camat L. A.; Castagnoli D.; Chuang N.; Chung L. N.; Do T.; Huynh E.; Jenpichitkulchai T.; Kaur A.; Le F.; Ong R.; Pham D.; Shao K. Model binding experiments with cucurbit[7]uril and p-sulfonatocalix[4]arene support use of explicit solvation term in governing equation for binding equilibria. Supramol. Chem. 2023, 34 (2), 94–104. 10.1080/10610278.2023.2254442. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
