Sensory input to cortex encoded on low-dimensional periphery-correlated subspaces
- PMID: 38250515
- PMCID: PMC10798852
- DOI: 10.1093/pnasnexus/pgae010
Sensory input to cortex encoded on low-dimensional periphery-correlated subspaces
Abstract
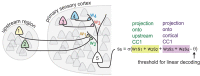
As information about the world is conveyed from the sensory periphery to central neural circuits, it mixes with complex ongoing cortical activity. How do neural populations keep track of sensory signals, separating them from noisy ongoing activity? Here, we show that sensory signals are encoded more reliably in certain low-dimensional subspaces. These coding subspaces are defined by correlations between neural activity in the primary sensory cortex and upstream sensory brain regions; the most correlated dimensions were best for decoding. We analytically show that these correlation-based coding subspaces improve, reaching optimal limits (without an ideal observer), as noise correlations between cortex and upstream regions are reduced. We show that this principle generalizes across diverse sensory stimuli in the olfactory system and the visual system of awake mice. Our results demonstrate an algorithm the cortex may use to multiplex different functions, processing sensory input in low-dimensional subspaces separate from other ongoing functions.
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures




References
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

