Analysis of CVC1302-Mediated Enhancement of Monocyte Recruitment in Inducing Immune Responses
- PMID: 38250899
- PMCID: PMC10820601
- DOI: 10.3390/vaccines12010086
Analysis of CVC1302-Mediated Enhancement of Monocyte Recruitment in Inducing Immune Responses
Abstract
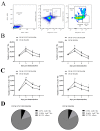
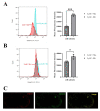
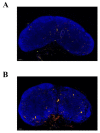
Monocytes (Mos) are believed to play important roles during the generation of immune response. In our previous study, CVC1302, a complex of PRRs agonists, was demonstrated to recruit Mo into lymph nodes (LNs) in order to present antigen and secret chemokines (CXCL9 and CXCL10), which attracted antigen-specific CD4+ T cells. As it is known that Mos in mice are divided into two main Mo subsets (Ly6C+ Mo and Ly6C- Mo), we aimed to clarify the CVC1302-recruiting Mo subset and functions in the establishment of immunity. In this study, we found that CVC1302 attracted both Ly6C+ Mo and Ly6C- Mo into draining LNs, which infiltrated from different origins, injection muscles and high endothelial venule (HEV), respectively. We also found that the numbers of OVA+ Ly6C+ Mo in the draining LNs were significantly higher compared with OVA+ Ly6C- Mo. However, the levels of CXCL9 and CXCL10 produced by Ly6C- Mo were significantly higher than Ly6C+ Mo, which plays important roles in attracting antigen-specific CD4+ T cells. Under the analysis of their functions in initiating immune responses, we found that the ability of the Ly6C+ monocyte was mainly capturing and presenting antigens, otherwise; the ability of the Ly6C- monocyte was mainly secreting CXCL9 and CXCL10, which attracted antigen-specific CD4+ T cells through CXCR3. These results will provide new insights into the development of new immunopotentiators and vaccines.
Keywords: CVC1302; CXCL10; CXCL9; antigen; monocyte.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Conventional dendritic cells 2, the targeted antigen-presenting-cell, induces enhanced type 1 immune responses in mice immunized with CVC1302 in oil formulation.Immunol Lett. 2024 Jun;267:106856. doi: 10.1016/j.imlet.2024.106856. Epub 2024 Mar 25. Immunol Lett. 2024. PMID: 38537718
-
Pattern-Recognition Receptor Agonist-Containing Immunopotentiator CVC1302 Boosts High-Affinity Long-Lasting Humoral Immunity.Front Immunol. 2021 Nov 18;12:697292. doi: 10.3389/fimmu.2021.697292. eCollection 2021. Front Immunol. 2021. PMID: 34867941 Free PMC article.
-
Identification of mechanisms conferring an enhanced immune response in mice induced by CVC1302-adjuvanted killed serotype O foot-and-mouth virus vaccine.Vaccine. 2019 Oct 8;37(43):6362-6370. doi: 10.1016/j.vaccine.2019.09.014. Epub 2019 Sep 13. Vaccine. 2019. PMID: 31526618
-
CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond.Front Immunol. 2020 May 29;11:976. doi: 10.3389/fimmu.2020.00976. eCollection 2020. Front Immunol. 2020. PMID: 32547545 Free PMC article. Review.
-
Monocyte-derived dendritic cells in malaria.Curr Opin Microbiol. 2019 Dec;52:139-150. doi: 10.1016/j.mib.2019.08.002. Epub 2019 Sep 19. Curr Opin Microbiol. 2019. PMID: 31542508 Free PMC article. Review.
References
-
- Patel A.A., Zhang Y., Fullerton J.N., Boelen L., Rongvaux A., Maini A.A., Bigley V., Flavell R.A., Gilroy D.W., Asquith B., et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017;214:1913–1923. doi: 10.1084/jem.20170355. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

