A Potential Association between Abdominal Obesity and the Efficacy of Humoral Immunity Induced by COVID-19 and by the AZD1222, Convidecia, BNT162b2, Sputnik V, and CoronaVac Vaccines
- PMID: 38250901
- PMCID: PMC10819553
- DOI: 10.3390/vaccines12010088
A Potential Association between Abdominal Obesity and the Efficacy of Humoral Immunity Induced by COVID-19 and by the AZD1222, Convidecia, BNT162b2, Sputnik V, and CoronaVac Vaccines
Abstract
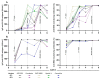
Abdominal obesity is highly prevalent in Mexico and has a poor prognosis in terms of the severity of coronavirus disease (COVID-19) and low levels of antibodies induced by infection and vaccination. We evaluated the humoral immune response induced by COVID-19 and five different vaccination schedules in Mexican individuals with abdominal obesity and the effects of other variables. This prospective longitudinal cohort study included 2084 samples from 389 participants. The levels of anti-S1/S2 and anti-RBD IgG antibodies were measured at various time points after vaccination. A high prevalence of hospitalization and oxygen use was observed in individuals with abdominal obesity (AO) who had COVID-19 before vaccination; however, they also had high levels of anti-S1/S2 and anti-RBD-neutralizing IgG antibodies. The same was true for vaccination-induced antibody levels. However, their longevity was low. Interestingly, we did not observe significant differences in vaccine reactogenicity between abdominally obese and abdominally non-obese groups. Finally, individuals with a higher body mass index, older age, and previous COVID-19 had higher levels of antibodies induced by COVID-19 and vaccination. Therefore, it is important to evaluate other immunological and inflammatory factors to better understand the pathogenesis of COVID-19 in the presence of risk factors and to propose effective vaccination schedules for vulnerable populations.
Keywords: COVID-19; abdominal obesity; humoral immune response; vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Obesity and Overweight. [(accessed on 2 November 2023)]. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight.
-
- Barquera S., Hernández-Barrera L., Trejo-Valdivia B., Shamah T., Campos-Nonato I., Rivera-Dommarco J. Obesidad en México, prevalencia y tendencias en adultos. Ensanut 2018-19 [Obesity in Mexico, prevalence andtrends in adults. Ensanut 2018-19] Salud Publica Mex. 2020;62:682–692. doi: 10.21149/11630. - DOI - PubMed
LinkOut - more resources
Full Text Sources

