Development of Cross-Reactive Live Attenuated Influenza Vaccine Candidates against Both Lineages of Influenza B Virus
- PMID: 38250908
- PMCID: PMC10821225
- DOI: 10.3390/vaccines12010095
Development of Cross-Reactive Live Attenuated Influenza Vaccine Candidates against Both Lineages of Influenza B Virus
Abstract
Background: Influenza viruses continue to cause a significant social and economic burden globally. Vaccination is recognized as the most effective measure to control influenza. Live attenuated influenza vaccines (LAIVs) are an effective means of preventing influenza, especially among children. A reverse genetics (RG) system is required to rapidly update the antigenic composition of vaccines, as well as to design LAIVs with a broader spectrum of protection. Such a system has been developed for the Russian LAIVs only for type A strains, but not for influenza B viruses (IBV).
Methods: All genes of the B/USSR/60/69 master donor virus (B60) were cloned into RG plasmids, and the engineered B60, as well as a panel of IBV LAIV reassortants were rescued from plasmid DNAs encoding all viral genes. The engineered viruses were evaluated in vitro and in a mouse model.
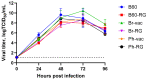
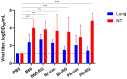
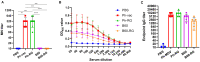
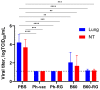
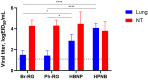
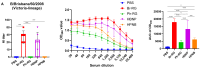
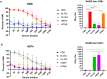
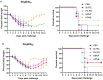
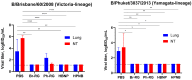
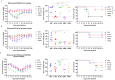
Results: The B60 RG system was successfully developed, which made it possible to rescue LAIV reassortants with the desired antigenic composition, including hybrid strains with hemagglutinin and neuraminidase genes belonging to the viruses from different IBV lineages. The LAIV candidate carrying the HA of the B/Victoria-lineage virus and NA from the B/Yamagata-lineage virus demonstrated optimal characteristics in terms of safety, immunogenicity and cross-protection, prompting its further assessment as a broadly protective component of trivalent LAIV.
Conclusions: The new RG system for B60 MDV allowed the rapid generation of type B LAIV reassortants with desired genome compositions. The generation of hybrid LAIV reassortants with HA and NA genes belonging to the opposite IBV lineages is a promising approach for the development of IBV vaccines with broad cross-protection.
Keywords: cross-protection; influenza B virus; live attenuated influenza vaccine; recombinant influenza virus; reverse genetics; viral immunity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Development of an Alternative Modified Live Influenza B Virus Vaccine.J Virol. 2017 May 26;91(12):e00056-17. doi: 10.1128/JVI.00056-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381580 Free PMC article.
-
Generation of DelNS1 Influenza Viruses: a Strategy for Optimizing Live Attenuated Influenza Vaccines.mBio. 2019 Sep 17;10(5):e02180-19. doi: 10.1128/mBio.02180-19. mBio. 2019. PMID: 31530680 Free PMC article.
-
A Live Attenuated Influenza Vaccine Elicits Enhanced Heterologous Protection When the Internal Genes of the Vaccine Are Matched to Those of the Challenge Virus.J Virol. 2020 Jan 31;94(4):e01065-19. doi: 10.1128/JVI.01065-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31748399 Free PMC article.
-
Novel Approaches for The Development of Live Attenuated Influenza Vaccines.Viruses. 2019 Feb 22;11(2):190. doi: 10.3390/v11020190. Viruses. 2019. PMID: 30813325 Free PMC article. Review.
-
Influenza B virus neuraminidase: a potential target for next-generation vaccines?Expert Rev Vaccines. 2024 Jan-Dec;23(1):39-48. doi: 10.1080/14760584.2023.2290691. Epub 2023 Dec 14. Expert Rev Vaccines. 2024. PMID: 38037386 Review.
Cited by
-
Safety, Immunogenicity and Protective Activity of a Modified Trivalent Live Attenuated Influenza Vaccine for Combined Protection Against Seasonal Influenza and COVID-19 in Golden Syrian Hamsters.Vaccines (Basel). 2024 Nov 21;12(12):1300. doi: 10.3390/vaccines12121300. Vaccines (Basel). 2024. PMID: 39771962 Free PMC article.
-
Harnessing T-Cells for Enhanced Vaccine Development against Viral Infections.Vaccines (Basel). 2024 Apr 29;12(5):478. doi: 10.3390/vaccines12050478. Vaccines (Basel). 2024. PMID: 38793729 Free PMC article. Review.
-
Genomic evolution of influenza during the 2023-2024 season, the johns hopkins health system.J Clin Virol. 2024 Oct;174:105718. doi: 10.1016/j.jcv.2024.105718. Epub 2024 Jul 25. J Clin Virol. 2024. PMID: 39079210
-
Extinction of influenza B Yamagata: Its impact on public health and vaccine implications.J Biomed Res. 2024 Aug 22;39(2):209-212. doi: 10.7555/JBR.38.20240158. J Biomed Res. 2024. PMID: 39164195 Free PMC article. No abstract available.
References
-
- Alexandrova G.I., Maassab H.F., Kendal A.P., Medvedeva T.E., Egorov A.Y., Klimov A.I., Cox N.J. Laboratory properties of cold-adapted influenza B live vaccine strains developed in the US and USSR, and their B/Ann Arbor/1/86 cold-adapted reassortant vaccine candidates. Vaccine. 1990;8:61–64. doi: 10.1016/0264-410X(90)90179-P. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

