All Eyes on the Prefusion-Stabilized F Construct, but Are We Missing the Potential of Alternative Targets for Respiratory Syncytial Virus Vaccine Design?
- PMID: 38250910
- PMCID: PMC10819635
- DOI: 10.3390/vaccines12010097
All Eyes on the Prefusion-Stabilized F Construct, but Are We Missing the Potential of Alternative Targets for Respiratory Syncytial Virus Vaccine Design?
Abstract
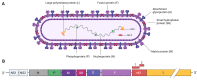
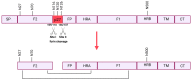
Respiratory Syncytial Virus (RSV) poses a significant global health concern as a major cause of lower respiratory tract infections (LRTIs). Over the last few years, substantial efforts have been directed towards developing vaccines and therapeutics to combat RSV, leading to a diverse landscape of vaccine candidates. Notably, two vaccines targeting the elderly and the first maternal vaccine have recently been approved. The majority of the vaccines and vaccine candidates rely solely on a prefusion-stabilized conformation known for its highly neutralizing epitopes. Although, so far, this antigen design appears to be successful for the elderly, our current understanding remains incomplete, requiring further improvement and refinement in this field. Pediatric vaccines still have a long journey ahead, and we must ensure that vaccines currently entering the market do not lose efficacy due to the emergence of mutations in RSV's circulating strains. This review will provide an overview of the current status of vaccine designs and what to focus on in the future. Further research into antigen design is essential, including the exploration of the potential of alternative RSV proteins to address these challenges and pave the way for the development of novel and effective vaccines, especially in the pediatric population.
Keywords: RSV; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A monomeric uncleaved respiratory syncytial virus F antigen retains prefusion-specific neutralizing epitopes.J Virol. 2014 Oct;88(20):11802-10. doi: 10.1128/JVI.01225-14. Epub 2014 Jul 30. J Virol. 2014. PMID: 25078705 Free PMC article.
-
Alternative Virus-Like Particle-Associated Prefusion F Proteins as Maternal Vaccines for Respiratory Syncytial Virus.J Virol. 2019 Nov 13;93(23):e00914-19. doi: 10.1128/JVI.00914-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511382 Free PMC article.
-
Rational design of a highly immunogenic prefusion-stabilized F glycoprotein antigen for a respiratory syncytial virus vaccine.Sci Transl Med. 2023 Apr 26;15(693):eade6422. doi: 10.1126/scitranslmed.ade6422. Epub 2023 Apr 26. Sci Transl Med. 2023. PMID: 37023209
-
The third pandemic: The respiratory syncytial virus landscape and specific considerations for the allergist/immunologist.Allergy Asthma Proc. 2023 Jul 26;44(4):220-228. doi: 10.2500/aap.2023.44.230030. Allergy Asthma Proc. 2023. PMID: 37236777 Review.
-
Current progress on development of respiratory syncytial virus vaccine.BMB Rep. 2011 Apr;44(4):232-7. doi: 10.5483/BMBRep.2011.44.4.232. BMB Rep. 2011. PMID: 21524347 Review.
Cited by
-
Immune Responses and Protection Profiles in Mice Induced by Subunit Vaccine Candidates Based on the Extracellular Domain Antigen of Respiratory Syncytial Virus G Protein Combined with Different Adjuvants.Vaccines (Basel). 2024 Jun 19;12(6):686. doi: 10.3390/vaccines12060686. Vaccines (Basel). 2024. PMID: 38932414 Free PMC article.
-
Whole-Genome Sequencing and Genetic Diversity of Human Respiratory Syncytial Virus in Patients with Influenza-like Illness in Sicily (Italy) from 2017 to 2023.Viruses. 2024 May 26;16(6):851. doi: 10.3390/v16060851. Viruses. 2024. PMID: 38932144 Free PMC article.
-
Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024).Vaccines (Basel). 2024 Jun 8;12(6):640. doi: 10.3390/vaccines12060640. Vaccines (Basel). 2024. PMID: 38932369 Free PMC article. Review.
-
Preventing RSV Infection in Children: Current Passive Immunizations and Vaccine Development.Pathogens. 2025 Jan 21;14(2):104. doi: 10.3390/pathogens14020104. Pathogens. 2025. PMID: 40005481 Free PMC article. Review.
-
Immunogenicity of RSV Fusion Protein Adsorbed to Non-Pathogenic Bacillus subtilis Spores: Implications for Mucosal Vaccine Delivery in Nonclinical Animal Models.Biomedicines. 2025 May 3;13(5):1112. doi: 10.3390/biomedicines13051112. Biomedicines. 2025. PMID: 40426939 Free PMC article.
References
-
- Shi T., McAllister D.A., O’Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

