A Practical Framework for Novel Electronic Nicotine Delivery System Evaluation: Chemical and Toxicological Characterization of JUUL2 Aerosol and Comparison with Reference Cigarettes
- PMID: 38250996
- PMCID: PMC10820849
- DOI: 10.3390/toxics12010041
A Practical Framework for Novel Electronic Nicotine Delivery System Evaluation: Chemical and Toxicological Characterization of JUUL2 Aerosol and Comparison with Reference Cigarettes
Abstract
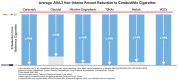
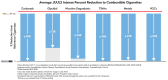
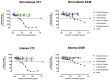
Electronic nicotine delivery systems (ENDSs) are designed as a non-combustible alternative to cigarettes, aiming to deliver nicotine without the harmful byproducts of tobacco combustion. As the category evolves and new ENDS products emerge, it is important to continually assess the levels of toxicologically relevant chemicals in the aerosols and characterize any related toxicology. Herein, we present a proposed framework for characterizing novel ENDS products (i.e., devices and formulations) and determining the reduced risk potential utilizing analytical chemistry and in vitro toxicological studies with a qualitative risk assessment. To demonstrate this proposed framework, long-term stability studies (12 months) analyzing relevant toxicant emissions from six formulations of a next-generation product, JUUL2, were conducted and compared to reference combustible cigarette (CC) smoke under both non-intense and intense puffing regimes. In addition, in vitro cytotoxicity, mutagenicity, and genotoxicity assays were conducted on aerosol and smoke condensates. In all samples, relevant toxicants under both non-intense and intense puffing regimes were substantially lower than those observed in reference CC smoke. Furthermore, neither cytotoxicity, mutagenicity, nor genotoxicity was observed in aerosol condensates generated under both intense and non-intense puffing regimes, in contrast to results observed for reference cigarettes. Following the proposed framework, the results demonstrate that the ENDS products studied in this work generate significantly lower levels of toxicants relative to reference cigarettes and were not cytotoxic, mutagenic, or genotoxic under these in vitro assay conditions.
Keywords: ENDS; aerosol; chemical; e-cigarette; electronic cigarette; framework; toxicological.
Conflict of interest statement
At the time this work was performed, all authors were employed by Juul Labs, Inc. All testing was performed at Enthalpy Analytical LLC and Charles River Laboratories and paid for in full by Juul Labs, Inc.
Figures

References
-
- U.S. Department of Health and Human Services . The Health Consequences of Smoking: A Report of the Surgeon General. Department of Health and Human Services; Atlanta, GA, USA: 2004. Reports of the Surgeon General; p. 956.
-
- Rodgman A., Perfetti T.A. The Chemical Components of Tobacco and Tobacco Smoke. 2nd ed. CRC Press; Boca Raton, FL, USA: 2013. p. 2332.
-
- U. S. Food and Drug Administration FDA’s Youth Tobacco Prevention Plan; [Web Page] 14 September 2020. [(accessed on 20 December 2023)]; Available online: https://www.fda.gov/tobacco-products/youth-and-tobacco/fdas-youth-tobacc....
-
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . Preventing Tobacco Use among Youth and Young Adults: A Report of the Surgeon General. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2012. - PubMed
-
- H.R. 1256–111th Congress (2009–2010) Family Smoking Prevention and Tobacco Control Act, in 21 U.S.C. § 301. Congress.gov, Library of Congress; Washington, DC, USA: 2009. p. 21.
LinkOut - more resources
Full Text Sources
Research Materials

