Toxicity Assessment of Mixed Exposure of Nine Perfluoroalkyl Substances at Concentrations Relevant to Daily Intake
- PMID: 38251008
- PMCID: PMC10819949
- DOI: 10.3390/toxics12010052
Toxicity Assessment of Mixed Exposure of Nine Perfluoroalkyl Substances at Concentrations Relevant to Daily Intake
Abstract
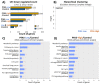
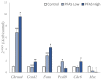
Per- and poly-fluoroalkyl substances (PFAS) exhibit high persistence in the environment and accumulate within the human body, warranting a thorough assessment of their toxicity. In this study, we exposed mice (male C57BL/6J mice aged 8 weeks) to a composite of nine PFAS, encompassing both long-chain PFAS (e.g., perfluorooctanoic acid and perfluorooctanesulfonic acid) and short-chain PFAS (e.g., perfluorobutanoic acid and perfluorobutanesulfonic acid). The exposure concentrations of PFAS were equivalent to the estimated daily human intake in the composition reported (1 µg/L (sum of the nine compounds), representing the maximum reported exposure concentration). Histological examination revealed hepatocyte vacuolization and irregular hepatocyte cord arrangement, indicating that exposure to low levels of the PFAS mixture causes morphological changes in liver tissues. Transcriptome analysis revealed that PFAS exposure mainly altered a group of genes related to metabolism and chemical carcinogenesis. Machine learning analysis of the liver metabolome showed a typical concentration-independent alteration upon PFAS exposure, with the annotation of substances such as glutathione and 5-aminovaleric acid. This study demonstrates that daily exposure to PFAS leads to morphological changes in liver tissues and alters the expression of metabolism- and cancer-related genes as well as phospholipid metabolism.
Keywords: environmentally relevant exposure; liver toxicity; multi-omics analysis; poly-fluoroalkyl substances.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury.Environ Int. 2021 Dec;157:106843. doi: 10.1016/j.envint.2021.106843. Epub 2021 Aug 31. Environ Int. 2021. PMID: 34479135 Free PMC article.
-
High-Throughput Transcriptomic Analysis of Human Primary Hepatocyte Spheroids Exposed to Per- and Polyfluoroalkyl Substances as a Platform for Relative Potency Characterization.Toxicol Sci. 2021 May 27;181(2):199-214. doi: 10.1093/toxsci/kfab039. Toxicol Sci. 2021. PMID: 33772556
-
Toxicity of perfluoroalkyl substances (PFAS) toward embryonic stages of mahi-mahi (Coryphaena hippurus).Ecotoxicology. 2022 Sep;31(7):1057-1067. doi: 10.1007/s10646-022-02576-w. Epub 2022 Aug 18. Ecotoxicology. 2022. PMID: 35982347
-
What are the effects of PFAS exposure at environmentally relevant concentrations?Chemosphere. 2020 Nov;258:127340. doi: 10.1016/j.chemosphere.2020.127340. Epub 2020 Jun 12. Chemosphere. 2020. PMID: 32563917 Review.
-
Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects.J Hazard Mater. 2021 Oct 5;419:126361. doi: 10.1016/j.jhazmat.2021.126361. Epub 2021 Jun 8. J Hazard Mater. 2021. PMID: 34157464 Review.
Cited by
-
Closing the Gaps in Understanding PFAS Toxicology and Metabolism.Toxics. 2024 Dec 27;13(1):19. doi: 10.3390/toxics13010019. Toxics. 2024. PMID: 39853019 Free PMC article.
References
-
- Buck R.C., Franklin J., Berger U., Conder J.M., Cousins I.T., De Voogt P., Jensen A.A., Kannan K., Mabury S.A., van Leeuwen S.P.J. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011;7:513–541. doi: 10.1002/ieam.258. - DOI - PMC - PubMed
-
- David Kempisty E.M., Racz L. Forever Chemicals: Environmental, Economic, and Social Equity Concerns with PFAS in the Environment. CRC Press; Boca Raton, FL, USA: 2021.
-
- Stohler S. Amazon Announces Ban on Toxic Chemicals and Plastics in Food Packaging. [(accessed on 9 November 2022)]. Available online: https://toxicfreefuture.org/press-room/amazon-announces-ban-on-toxic-che...
-
- Olsen G.W., Burris J.M., Ehresman D.J., Froelich J.W., Seacat A.M., Butenhoff J.L., Zobel L.R. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ. Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

