A Phase I, Randomized, SAD, MAD, and PK Study of Risvodetinib in Older Adults and Parkinson's Disease
- PMID: 38251063
- PMCID: PMC10977428
- DOI: 10.3233/JPD-230319
A Phase I, Randomized, SAD, MAD, and PK Study of Risvodetinib in Older Adults and Parkinson's Disease
Abstract
Background: Pre-clinical studies suggest that c-Abl activation may play an important role in the etiology of Parkinson's disease, making c-Abl an important target to evaluate for potential disease-modification.
Objective: To assess safety, tolerability, and pharmacokinetics of the c-Abl inhibitor risvodetinib (IkT-148009) in healthy subjects and participants with Parkinson's disease.
Methods: Part 1 (single ascending dose (SAD)) and Part 2 (7-day multiple ascending dose (MAD)) studies were in healthy volunteers. Participants were randomized 3 : 1 across 9 SAD doses and 3 MAD doses of risvodetinib (IkT-148009) or placebo. Part 3 was a MAD study conducted at two doses in 14 participants with mild-to-moderate PD (MAD-PD). Primary outcome measures were safety, tolerability and pharmacokinetics. Exploratory outcomes in PD participants included clinical measures of PD state, GI function, and cerebrospinal fluid (CSF) concentration.
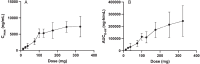
Results: 108 patients were treated with no dropouts. The SAD tested doses ranging from 12.5 to 325 mg, while the MAD tested 25 to 200 mg and MAD-PD tested 50 to 100 mg in Parkinson's participants. All active doses had a favorable safety profile with no clinically meaningful adverse events. Single dose pharmacokinetics were approximately linear between 12.5 mg and 200 mg for both Cmax and AUC0 - inf without distinction between healthy volunteers and participants with PD. Exposures at each dose were high relative to other drugs in the same kinase inhibitor class.
Conclusions: Risvodetinib (IkT-148009) was well tolerated, had a favorable safety and pharmacology profile over 7-day dosing, did not induce serious adverse events and did not appear to induce deleterious side-effects in participants administered anti-PD medications.
Keywords: Parkinson’s disease; c-Abl inhibition; disease-modification; neurodegeneration; pharmacology; therapeutics.
Conflict of interest statement
M.H.W. is the Founder, Chief Executive and Largest Shareholder of Inhibikase Therapeutics, Inc. C.M., C.K. and J.P. are employees and shareholders of Inhibikase Therapeutics, Inc. C.W.O is Chief Executive of Clintrex Research Corporation which provides services for many pharmaceutical and biotech companies including Inhibikase, is a shareholder in Inhibikase and serves as an expert witness in the paraquat litigation.
Figures

References
-
- Bach JP, Ziegler U, Deuschl G, Dodel R, Doblhammer-Reiter G (2011) Projected numbers of people with movement disorders in the years 2030 and 2050. Mov Disord 26, 2286–2290. - PubMed
-
- Pirooznia SK, Rosenthal LS, Dawson VL, Dawson TM (2021) Parkinson disease: translating insights from molecular mechanisms to neuroprotection. Pharmacol Rev 73, 33–97. - PubMed
-
- Goedert M (2001) Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2, 492–501. - PubMed
-
- Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9, 13–24. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

