The Effect of Adding CeO2 Nanoparticles to Cu-Ni-Al Alloy for High Temperatures Applications
- PMID: 38251108
- PMCID: PMC10820015
- DOI: 10.3390/nano14020143
The Effect of Adding CeO2 Nanoparticles to Cu-Ni-Al Alloy for High Temperatures Applications
Abstract
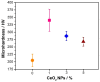
This work presents the effect of CeO2 nanoparticles (CeO2-NPs) on Cu-50Ni-5Al alloys on morphological, microstructural, degradation, and electrochemical behavior at high temperatures. The samples obtained by mechanical alloying and spark plasma sintering were exposed to a molten eutectic mixture of Li2CO3-K2CO3 for 504 h. The degradation of the materials was analyzed using gravimetry measurements and electrochemical impedance spectroscopy. Different characterization techniques, such as X-ray diffraction and scanning electron microscopy, were used to investigate the phase composition, parameter lattice, and microstructure of Cu-Ni-Al alloys reinforced with CeO2-NPs. The hardness of the composite was also examined using the Vickers hardness test. Gravimetry measurements revealed that the sample with 1 wt.% CeO2-NPs presented the best response to degradation with a less drastic mass variation. Impedance analysis also revealed that by adding 1 wt.% CeO2-NPs, the impedance modulus increased, which is related to a lower porosity of the oxide film or a thicker oxide layer. The microhardness also significantly increased, incorporating 1 wt.% CeO2-NPs, which reduced with higher CeO2-NPs content, which is possibly associated with a more uniform distribution using 1 wt.% CeO2-NPs in the Cu-Ni-Al matrix that avoided the aggregation phenomenon.
Keywords: CeO2–NPs; Cu–Ni–Al; gravimetry measurements; high temperatures; impedance; microhardness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

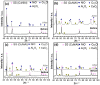
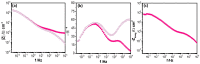
 ) 0 wt.% CeO2–NPs, (
) 0 wt.% CeO2–NPs, ( ) 1 wt.% CeO2–NPs, (
) 1 wt.% CeO2–NPs, ( ) 3 wt.% CeO2–NPs, (
) 3 wt.% CeO2–NPs, ( ) 5 wt.% CeO2–NPs.
) 5 wt.% CeO2–NPs.
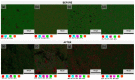
 ) 0% wt.% CeO2–NPs, (
) 0% wt.% CeO2–NPs, ( ) 1% wt.% CeO2–NPs, (
) 1% wt.% CeO2–NPs, ( ) 3% wt.% CeO2–NPs, and (
) 3% wt.% CeO2–NPs, and ( ) 5% wt.% CeO2–NPs before exposure.
) 5% wt.% CeO2–NPs before exposure.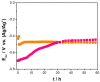
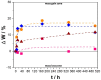
 ) Cu–50Ni–5Al and (
) Cu–50Ni–5Al and ( ) Cu–50Ni–5Al + 1 wt.% CeO2–NPs exposure to Li2CO3–K2CO3 at 550 °C and aerated atmosphere over time.
) Cu–50Ni–5Al + 1 wt.% CeO2–NPs exposure to Li2CO3–K2CO3 at 550 °C and aerated atmosphere over time.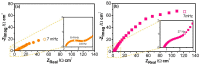
References
-
- Nanadegani F.S., Sunden B. Review of Exergy and Energy Analysis of Fuel Cells. Int. J. Hydrog. Energy. 2023 doi: 10.1016/j.ijhydene.2023.05.052. in press . - DOI
-
- Sharaf O.Z., Orhan M.F. An Overview of Fuel Cell Technology: Fundamentals and Applications. Renew. Sustain. Energy Rev. 2014;32:810–853. doi: 10.1016/j.rser.2014.01.012. - DOI
-
- Ming W., Sun P., Zhang Z., Qiu W., Du J., Li X., Zhang Y., Zhang G., Liu K., Wang Y., et al. A Systematic Review of Machine Learning Methods Applied to Fuel Cells in Performance Evaluation, Durability Prediction, and Application Monitoring. Int. J. Hydrog. Energy. 2023;48:5197–5228. doi: 10.1016/j.ijhydene.2022.10.261. - DOI
-
- Olabi A.G., Wilberforce T., Sayed E.T., Elsaid K., Abdelkareem M.A. Prospects of Fuel Cell Combined Heat and Power Systems. Energies. 2020;13:4104. doi: 10.3390/en13164104. - DOI
LinkOut - more resources
Full Text Sources

