The Synthesis of Sponge-like V2O5/CNT Hybrid Nanostructures Using Vertically Aligned CNTs as Templates
- PMID: 38251174
- PMCID: PMC10820936
- DOI: 10.3390/nano14020211
The Synthesis of Sponge-like V2O5/CNT Hybrid Nanostructures Using Vertically Aligned CNTs as Templates
Abstract
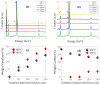
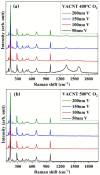
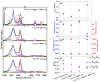
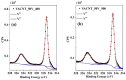
The fabrication of sponge-like vanadium pentoxide (V2O5) nanostructures using vertically aligned carbon nanotubes (VACNTs) as a template is presented. The VACNTs were grown on silicon substrates by chemical vapor deposition using the Fe/Al bilayer catalyst approach. The V2O5 nanostructures were obtained from the thermal oxidation of metallic vanadium deposited on the VACNTs. Different oxidation temperatures and vanadium thicknesses were used to study the influence of these parameters on the stability of the carbon template and the formation of the V2O5 nanostructures. The morphology of the samples was analyzed by scanning electron microscopy, and the structural characterization was performed by Raman, energy-dispersive X-ray, and X-ray photoelectron spectroscopies. Due to the catalytic properties of V2O5 in the decomposition of carbonaceous materials, it was possible to obtain supported sponge-like structures based on V2O5/CNT composites, in which the CNTs exhibit an increase in their graphitization. The VACNTs can be removed or preserved by modulating the thermal oxidation process and the vanadium thickness.
Keywords: chemical vapor deposition; electron beam deposition; thermal oxidation; vanadium pentoxide; vertically aligned carbon nanotubes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Schneider K., Lubecka M., Czapla A. V2O5 thin films for gas sensor applications. Sens. Actuators B Chem. 2016;236:970–977. doi: 10.1016/j.snb.2016.04.059. - DOI
-
- Vernardou D. Using an atmospheric pressure chemical vapor deposition process for the development of v2o5 as an electrochromic material. Coatings. 2017;7:24. doi: 10.3390/coatings7020024. - DOI
-
- Aliaga J., Cifuentes N., González G., Sotomayor-Torres C., Benavente E. Enhancement photocatalytic activity of the heterojunction of two-dimensional hybrid semiconductors ZnO/V2O5. Catalysts. 2018;8:374. doi: 10.3390/catal8090374. - DOI
-
- Ling H., Zhang R., Ye X., Wen Z., Xia J., Lu X. In-situ synthesis of organic-inorganic hybrid thin film of PEDOT/V2O5 as hole transport layer for polymer solar cells. Sol. Energy. 2019;190:63–68. doi: 10.1016/j.solener.2019.07.095. - DOI
-
- Yue Y., Liang H. Micro- and Nano-Structured Vanadium Pentoxide (V2O5) for Electrodes of Lithium-Ion Batteries. Adv. Energy Mater. 2017;7:1602545. doi: 10.1002/aenm.201602545. - DOI
Grants and funding
- Millennium Institute on Green Ammonia as Energy Vector MIGA, ANID/Millennium Science Initiative Program/ICN2021_023/Agencia Nacional de Investigación y Desarrollo
- FONDECYT 1201589/Agencia Nacional de Investigación y Desarrollo
- National Doctoral Scholarship CONICYT 21171760/Agencia Nacional de Investigación y Desarrollo
- FONDEQUIP EQM140142/Agencia Nacional de Investigación y Desarrollo
- FONDEQUIP EQM150101/Agencia Nacional de Investigación y Desarrollo
LinkOut - more resources
Full Text Sources

