Grafting Electron-Accepting Fragments on [4]cyclo-2,7-carbazole Scaffold: Tuning the Structural and Electronic Properties of Nanohoops
- PMID: 38251412
- PMCID: PMC10987112
- DOI: 10.1002/advs.202309115
Grafting Electron-Accepting Fragments on [4]cyclo-2,7-carbazole Scaffold: Tuning the Structural and Electronic Properties of Nanohoops
Abstract
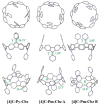
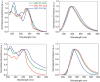
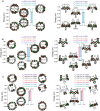
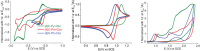
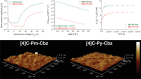
Since the first applications of nanohoops in organic electronics appear promising, the time has come to go deeper into their rational design in order to reach high-efficiency materials. To do so, systematic studies dealing with the incorporation of electron-rich and/or electron-poor functional units on nanohoops have to be performed. Herein, the synthesis, the electrochemical, photophysical, thermal, and structural properties of two [4]cyclo-2,7-carbazoles, [4]C-Py-Cbz, and [4]C-Pm-Cbz, possessing electron-withdrawing units on their nitrogen atoms (pyridine or pyrimidine) are reported. The synthesis of these nanohoops is first optimized and a high yield above 50% is reached. Through a structure-properties relationship study, it is shown that the substituent has a significant impact on some physicochemical properties (eg HOMO/LUMO levels) while others are kept unchanged (eg fluorescence). Incorporation in electronic devices shows that the most electrically efficient Organic Field-Effect transistors are obtained with [4]C-Py-Cbz although this compound does not present the best-organized semiconductor layer. These experimental data are finally confronted with the electronic couplings between the nanohoops determined at the DFT level and have highlighted the origin in the difference of charge transport properties. [4]C-Py-Cbz has the advantage of a more 2D-like transport character than [4]C-Pm-Cbz, which alleviates the impact of defects and structural organization.
Keywords: bridged cyclo‐oligophenylenes; charge transport; nanohoops; organic electronics; organic semiconductors.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
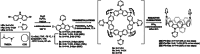




References
-
- Brouillac C., Lucas F., Tondelier D., Rault‐Berthelot J., Lebreton C., Jacques E., Quinton C., Poriel C., Adv. Opt. Mater. 2023, 11, 2202191.
-
- Ball M., Zhong Y., Fowler B., Zhang B., Li P., Etkin G., Paley D. W., Decatur J., Dalsania A. K., Li H., Xiao S., Ng F., Steigerwald M. L., Nuckolls C., J. Am. Chem. Soc. 2016, 138, 12861. - PubMed
-
- Sicard L., Jeannin O., Rault‐Berthelot J., Quinton C., Poriel C., ChemPlusChem 2018, 83, 874. - PubMed
-
- Zhang B., Trinh M. T., Fowler B., Ball M., Xu Q., Ng F., Steigerwald M. L., Zhu X.‐Y., Nuckolls C., Zhong Y., J. Am. Chem. Soc. 2016, 16426. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
