Clinical and electrophysiological features of SCN8A variants causing episodic or chronic ataxia
- PMID: 38251463
- PMCID: PMC10628346
- DOI: 10.1016/j.ebiom.2023.104855
Clinical and electrophysiological features of SCN8A variants causing episodic or chronic ataxia
Abstract
Background: Variants in SCN8A are associated with a spectrum of epilepsies and neurodevelopmental disorders. Ataxia as a predominant symptom of SCN8A variation has not been well studied. We set out to investigate disease mechanisms and genotype-phenotype correlations of SCN8A-related ataxia.
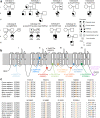
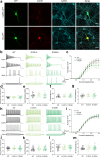
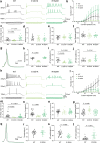
Methods: We collected genetic and electro-clinical data of ten individuals from nine unrelated families carrying novel SCN8A variants associated with chronic progressive or episodic ataxia. Electrophysiological characterizations of these variants were performed in ND7/23 cells and cultured neurons.
Findings: Variants associated with chronic progressive ataxia either decreased Na+ current densities and shifted activation curves towards more depolarized potentials (p.Asn995Asp, p.Lys1498Glu and p.Trp1266Cys) or resulted in a premature stop codon (p.Trp937Ter). Three variants (p.Arg847Gln and biallelic p.Arg191Trp/p.Asp1525Tyr) were associated with episodic ataxia causing loss-of-function by decreasing Na+ current densities or a hyperpolarizing shift of the inactivation curve. Two additional episodic ataxia-associated variants caused mixed gain- and loss-of function effects in ND7/23 cells and were further examined in primary murine hippocampal neuronal cultures. Neuronal firing in excitatory neurons was increased by p.Arg1629His, but decreased by p.Glu1201Lys. Neuronal firing in inhibitory neurons was decreased for both variants. No functional effect was observed for p.Arg1913Trp. In four individuals, treatment with sodium channel blockers exacerbated symptoms.
Interpretation: We identified episodic or chronic ataxia as predominant phenotypes caused by variants in SCN8A. Genotype-phenotype correlations revealed a more pronounced loss-of-function effect for variants causing chronic ataxia. Sodium channel blockers should be avoided under these conditions.
Funding: BMBF, DFG, the Italian Ministry of Health, University of Tuebingen.
Keywords: Chronic ataxia; Episodic ataxia; Loss-of-function; Patch-clamp; SCN8A.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests MK reports a doctoral grant from DAAD. JDOE is coordinator of the Chorea and Huntington Disease Group (ERN-RND). EB has received funding from the Dutch Epilepsy Foundation, on behalf of the Dravet syndrome Foundation Netherlands/Flanders, the JANIVO foundation and the K.F. Hein Foundation. LS declares grants from the German Research Foundation, German Ministry of Heath, European Commission, and Innovationsfond, and has received consulting fees from Vico Therapeutics and Novartis. RSM has received consulting and speaker fees from UCB and Orion and speaker fees from EISAI and Angelini Pharma. All other authors report no competing interests.
Figures

References
-
- Schule R., Schols L. [Ataxias and hereditary spastic paraplegias] Nervenarzt. 2017;88(7):720–727. - PubMed
-
- Jen J.C., Graves T.D., Hess E.J., et al. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain. 2007;130(Pt 10):2484–2493. - PubMed
-
- Hadjivassiliou M., Martindale J., Shanmugarajah P., et al. Causes of progressive cerebellar ataxia: prospective evaluation of 1500 patients. J Neurol Neurosurg Psychiatry. 2017;88(4):301–309. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

