Atomic-Scale Time-Resolved Imaging of Krypton Dimers, Chains and Transition to a One-Dimensional Gas
- PMID: 38251654
- PMCID: PMC10832048
- DOI: 10.1021/acsnano.3c07853
Atomic-Scale Time-Resolved Imaging of Krypton Dimers, Chains and Transition to a One-Dimensional Gas
Abstract
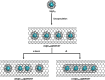
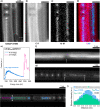
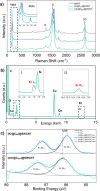
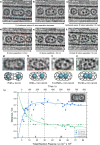
Single-atom dynamics of noble-gas elements have been investigated using time-resolved transmission electron microscopy (TEM), with direct observation providing for a deeper understanding of chemical bonding, reactivity, and states of matter at the nanoscale. We report on a nanoscale system consisting of endohedral fullerenes encapsulated within single-walled carbon nanotubes ((Kr@C60)@SWCNT), capable of the delivery and release of krypton atoms on-demand, via coalescence of host fullerene cages under the action of the electron beam (in situ) or heat (ex situ). The state and dynamics of Kr atoms were investigated by energy dispersive X-ray spectroscopy (EDS), electron energy loss spectroscopy (EELS), and X-ray photoelectron spectroscopy (XPS). Kr atom positions were measured precisely using aberration-corrected high-resolution TEM (AC-HRTEM), aberration-corrected scanning TEM (AC-STEM), and single-atom spectroscopic imaging (STEM-EELS). The electron beam drove the formation of 2Kr@C120 capsules, in which van der Waals Kr2 and transient covalent [Kr2]+ bonding states were identified. Thermal coalescence led to the formation of longer coalesced nested nanotubes containing more loosely bound Krn chains (n = 3-6). In some instances, delocalization of Kr atomic positions was confirmed by STEM analysis as the transition to a one-dimensional (1D) gas, as Kr atoms were constrained to only one degree of translational freedom within long, well-annealed, nested nanotubes. Such nested nanotube structures were investigated by Raman spectroscopy. This material represents a highly compressed and dimensionally constrained 1D gas stable under ambient conditions. Direct atomic-scale imaging has revealed elusive bonding states and a previously unseen 1D gaseous state of matter of this noble gas element, demonstrating TEM to be a powerful tool in the discovery of chemistry at the single-atom level.
Keywords: carbon nanotubes; endohedral fullerenes; noble gases; one-dimensional gas; single-atom dynamics; time-resolved imaging; transmission electron microscopy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
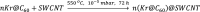

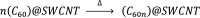
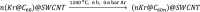



References
-
- Hernandez E.; Meunier V.; Smith B. W.; Rurali R.; Terrones H.; Nardelli M. B.; Terrones M.; Luzzi D. E.; Charlier J. Fullerene Coalescence in Nanopeapods: A Path to Novel Tubular Carbon. Nano Lett. 2003, 3 (8), 1037–1042. 10.1021/nl034283f. - DOI
-
- Smith B. W.; Luzzi D. E. Formation Mechanism of Fullerene Peapods and Coaxial Tubes: A Path to Large Scale Synthesis. Chem. Phys. Lett. 2000, 321 (1–2), 169–174. 10.1016/S0009-2614(00)00307-9. - DOI

