Race and Ethnic Group Enrollment and Outcomes for Wilms Tumor: Analysis of the Current Era Children's Oncology Group Study, AREN03B2
- PMID: 38251681
- PMCID: PMC11138877
- DOI: 10.1097/XCS.0000000000000999
Race and Ethnic Group Enrollment and Outcomes for Wilms Tumor: Analysis of the Current Era Children's Oncology Group Study, AREN03B2
Abstract
Background: To review race and ethnic group enrollment and outcomes for Wilms tumor (WT) across all 4 risk-assigned therapeutic trials from the current era Children's Oncology Group Renal Tumor Biology and Risk Stratification Protocol, AREN03B2.
Study design: For patients with WT enrolled in AREN03B2 (2006 to 2019), disease and biologic features, therapeutic study-specific enrollment, and event-free (EFS) and overall (OS) 4-year survival were compared between institutionally reported race and ethnic groups.
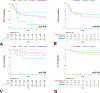
Results: Among 5,146 patients with WT, no statistically significant differences were detected between race and ethnic groups regarding subsequent risk-assigned therapeutic study enrollment, disease stage, histology, biologic factors, or overall EFS or OS, except the following variables: Black children were older and had larger tumors at enrollment, whereas Hispanic children had lower rates of diffuse anaplasia WT and loss of heterozygosity at 1p. The only significant difference in EFS or OS between race and ethnic groups was observed among the few children treated for diffuse anaplasia WT with regimen UH-1 and -2 on high-risk protocol, AREN0321. On this therapeutic arm only, Black children showed worse EFS (hazard ratio = 3.18) and OS (hazard ratio = 3.42). However, this finding was not replicated for patients treated with regimen UH-1 and -2 under AREN03B2 but not on AREN0321.
Conclusions: Race and ethnic group enrollment appeared constant across AREN03B2 risk-assigned therapeutic trials. EFS and OS on these therapeutic trials when analyzed together were comparable regarding race and ethnicity. Black children may have experienced worse stage-specific survival when treated with regimen UH-1 and -2 on AREN0321, but this survival gap was not confirmed when analyzing additional high-risk AREN03B2 patients.
Copyright © 2024 by the American College of Surgeons. Published by Wolters Kluwer Health, Inc. All rights reserved.
Figures





References
-
- Libes J, Hol J, Neto JCA, et al. Pediatric renal tumor epidemiology: Global perspectives, progress, and challenges. Pediatr Blood Cancer. 2023;70 Suppl 2:e30343. - PubMed
-
- Spreafico F, Fernandez CV, Brok J, et al. Wilms tumour. Nat Rev Dis Primers. 2021;7(1):75. - PubMed
-
- Dome JS, Perlman EJ, Graf N. Risk stratification for wilms tumor: current approach and future directions. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2014:215–23. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

