Structure of the two-component S-layer of the archaeon Sulfolobus acidocaldarius
- PMID: 38251732
- PMCID: PMC10903991
- DOI: 10.7554/eLife.84617
Structure of the two-component S-layer of the archaeon Sulfolobus acidocaldarius
Abstract
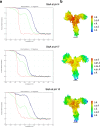
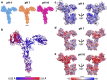
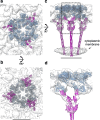
Surface layers (S-layers) are resilient two-dimensional protein lattices that encapsulate many bacteria and most archaea. In archaea, S-layers usually form the only structural component of the cell wall and thus act as the final frontier between the cell and its environment. Therefore, S-layers are crucial for supporting microbial life. Notwithstanding their importance, little is known about archaeal S-layers at the atomic level. Here, we combined single-particle cryo electron microscopy, cryo electron tomography, and Alphafold2 predictions to generate an atomic model of the two-component S-layer of Sulfolobus acidocaldarius. The outer component of this S-layer (SlaA) is a flexible, highly glycosylated, and stable protein. Together with the inner and membrane-bound component (SlaB), they assemble into a porous and interwoven lattice. We hypothesise that jackknife-like conformational changes in SlaA play important roles in S-layer assembly.
Keywords: S-layer; Sulfolobus; archaea; cryoEM; molecular biophysics; single-particle analysis; structural biology; sub-tomogram averaging; sulfolobus acidocaldarius; tomography.
© 2024, Gambelli et al.
Conflict of interest statement
LG, MM, RC, KS, MG, LC, VG, DK, MS, CH, MI, BD No competing interests declared
Figures


















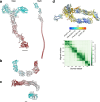

Update of
- doi: 10.1101/2022.10.07.511299
Comment in
- doi: 10.7554/eLife.96485
References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. doi: 10.1016/j.softx.2015.06.001. - DOI
-
- Abu-Qarn M, Yurist-Doutsch S, Giordano A, Trauner A, Morris HR, Hitchen P, Medalia O, Dell A, Eichler J. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. Journal of Molecular Biology. 2007;374:1224–1236. doi: 10.1016/j.jmb.2007.10.042. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

