Interfacial rheology of linearly growing polyelectrolyte multilayers at the water-air interface: from liquid to solid viscoelasticity
- PMID: 38252016
- PMCID: PMC10848651
- DOI: 10.1039/d3sm01161e
Interfacial rheology of linearly growing polyelectrolyte multilayers at the water-air interface: from liquid to solid viscoelasticity
Abstract
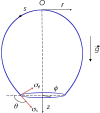
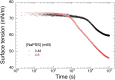
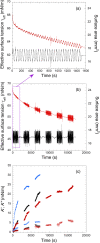
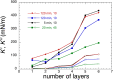
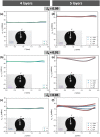
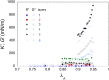
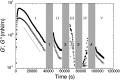
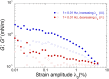
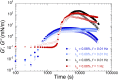
Despite the long history of investigations of polyelectrolyte multilayer formation on solid or liquid surfaces, important questions remain open concerning the construction of the first set of layers. These are generally deposited on a first anchoring layer of different chemistry, influencing their construction and properties. We propose here an in-depth investigation of the formation of NaPSS/PAH multilayers at the air/water interface in the absence of a chemically different anchoring layer, profiting from the surface activity of NaPSS. To analyse the mechanical properties of the different layers, we combine recently established analysis techniques of an inflating/deflating bubble exploiting simultaneous shape and pressure measurement: bubble shape elastometry, general stress decomposition and capillary meniscus dynanometry. We complement these measurements by interfacial shear rheology. The obtained results allow us to confirm, first of all, the strength of the aforementioned techniques to characterize complex interfaces with non-linear viscoelastic properties. Furthermore, their sensitivity allows us to show that the multilayer properties are highly sensitive to the temporal and mechanical conditions under which they are constructed and manipulated. We nevertheless identify a robust trend showing a clear transition from a liquid-like viscoelastic membrane to a solid-like viscoelastic membrane after the deposition of 5 layers. We interpret this as the number of layers required to create a fully connected multilayer, which is consistent with previous results obtained on solid or liquid interfaces.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Decher G. Hong J. D. Schmitt J. Thin Solid Films. 1992;210–211:831–835. doi: 10.1016/0040-6090(92)90417-A. - DOI
-
- Jaber J. A. Schlenoff J. B. Curr. Opin. Colloid Interface Sci. 2006;11:324–329. doi: 10.1016/j.cocis.2006.09.008. - DOI
-
- Shchukina E. M. Shchukin D. G. Curr. Opin. Colloid Interface Sci. 2012;17:281–289. doi: 10.1016/j.cocis.2012.06.003. - DOI
LinkOut - more resources
Full Text Sources

