Metastable States in the Hinge-Bending Landscape of an Enzyme in an Atomistic Cytoplasm Simulation
- PMID: 38252018
- PMCID: PMC11180962
- DOI: 10.1021/acs.jpclett.3c03134
Metastable States in the Hinge-Bending Landscape of an Enzyme in an Atomistic Cytoplasm Simulation
Abstract
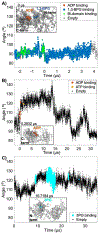
Many enzymes undergo major conformational changes to function in cells, particularly when they bind to more than one substrate. We quantify the large-amplitude hinge-bending landscape of human phosphoglycerate kinase (PGK) in a human cytoplasm. Approximately 70 μs of all-atom simulations, upon coarse graining, reveal three metastable states of PGK with different hinge angle distributions and additional substates. The "open" state was more populated than the "semi-open" or "closed" states. In addition to free energies and barriers within the landscape, we characterized the average transition state passage time of ≈0.3 μs and reversible substrate and product binding. Human PGK in a dilute solution simulation shows a transition directly from the open to closed states, in agreement with previous SAXS experiments, suggesting that the cell-like model environment promotes stability of the human PGK semi-open state. Yeast PGK also sampled three metastable states within the cytoplasm model, with the closed state favored in our simulation.
Figures




Similar articles
-
A bisubstrate analog induces unexpected conformational changes in phosphoglycerate kinase from Trypanosoma brucei.J Mol Biol. 1998 Jun 26;279(5):1137-48. doi: 10.1006/jmbi.1998.1835. J Mol Biol. 1998. PMID: 9642090
-
Conformational changes in yeast phosphoglycerate kinase upon substrate binding.Biophys Chem. 1994 Dec;53(1-2):95-104. doi: 10.1016/0301-4622(94)00080-8. Biophys Chem. 1994. PMID: 7841334
-
Crystal structures of substrates and products bound to the phosphoglycerate kinase active site reveal the catalytic mechanism.Biochemistry. 1998 Mar 31;37(13):4429-36. doi: 10.1021/bi9724117. Biochemistry. 1998. PMID: 9521762
-
Folding funnels and conformational transitions via hinge-bending motions.Cell Biochem Biophys. 1999;31(2):141-64. doi: 10.1007/BF02738169. Cell Biochem Biophys. 1999. PMID: 10593256 Review.
-
Denatured states of yeast phosphoglycerate kinase.Biochemistry (Mosc). 1998 Mar;63(3):259-75. Biochemistry (Mosc). 1998. PMID: 9526123 Review.
Cited by
-
Recent Progress in Modeling and Simulation of Biomolecular Crowding and Condensation Inside Cells.J Chem Inf Model. 2024 Dec 23;64(24):9063-9081. doi: 10.1021/acs.jcim.4c01520. Epub 2024 Dec 11. J Chem Inf Model. 2024. PMID: 39660892 Free PMC article. Review.
-
PEG-mCherry interactions beyond classical macromolecular crowding.Protein Sci. 2025 Mar;34(3):e5235. doi: 10.1002/pro.5235. Protein Sci. 2025. PMID: 39968832 Free PMC article.
-
Enzymes in a human cytoplasm model organize into submetabolon complexes.Proc Natl Acad Sci U S A. 2025 Feb 4;122(5):e2414206122. doi: 10.1073/pnas.2414206122. Epub 2025 Jan 28. Proc Natl Acad Sci U S A. 2025. PMID: 39874290 Free PMC article.
References
-
- Kumar S; Ma B; Tsai C-J; Wolfson H; Nussinov R Folding Funnels and Conformational Transitions via Hinge-Bending Motions. Cell Biochem. Biophys 1999, 31, 141–164. - PubMed
-
- Biehl R; Richter D Slow Internal Protein Dynamics in Solution. J. Phys. Condens. Matter Inst. Phys. J 2014, 26, 503103. - PubMed
-
- McCammon JA; Gelin BR; Karplus M; Wolynes PG The Hinge-Bending Mode in Lysozyme. Nature 1976, 262, 325–326. - PubMed
-
- Haran G; Mazal H How Fast Are the Motions of Tertiary-Structure Elements in Proteins? J. Chem. Phys 2020, 153, 130902. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

