Neurosteroids and their potential as a safer class of general anesthetics
- PMID: 38252143
- PMCID: PMC10954990
- DOI: 10.1007/s00540-023-03291-4
Neurosteroids and their potential as a safer class of general anesthetics
Abstract
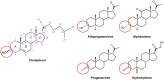
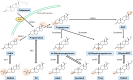
Neurosteroids (NS) are a class of steroids that are synthesized within the central nervous system (CNS). Various NS can either enhance or inhibit CNS excitability and they play important biological roles in brain development, brain function and as mediators of mood. One class of NS, 3α-hydroxy-pregnane steroids such as allopregnanolone (AlloP) or pregnanolone (Preg), inhibits neuronal excitability; these endogenous NS and their analogues have been therapeutically applied as anti-depressants, anti-epileptics and general anesthetics. While NS have many favorable properties as anesthetics (e.g. rapid onset, rapid recovery, minimal cardiorespiratory depression, neuroprotection), they are not currently in clinical use, largely due to problems with formulation. Recent advances in understanding NS mechanisms of action and improved formulations have rekindled interest in development of NS as sedatives and anesthetics. In this review, the synthesis of NS, and their mechanism of action will be reviewed with specific emphasis on their binding sites and actions on γ-aminobutyric acid type A (GABAA) receptors. The potential advantages of NS analogues as sedative and anesthetic agents will be discussed.
Keywords: Allopregnanolone; Alphaxalone; GABAA receptor; Neurosteroids.
© 2024. The Author(s).
Conflict of interest statement
The authors declared no conflict of interest.
Figures

References
-
- Selye H. Anesthetic Effect of Steroid Hormones. Proc Soc Exp Biol Med. 1941;46:116–121. doi: 10.3181/00379727-46-11907. - DOI
-
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, Hoffmann E, Rubinow D, Jonas J, Paul S, Meltzer-Brody S. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–489. doi: 10.1016/S0140-6736(17)31264-3. - DOI - PubMed
-
- Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, Li H, Sankoh AJ, Clemson C, Schacterle A, Jonas J, Kanes S. Brexanolone injection in post-partum depression: two multicentre, doubleblind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058–1070. doi: 10.1016/S0140-6736(18)31551-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

