FOXA2-initiated transcriptional activation of INHBA induced by methylmalonic acid promotes pancreatic neuroendocrine neoplasm progression
- PMID: 38252148
- PMCID: PMC10803496
- DOI: 10.1007/s00018-023-05084-0
FOXA2-initiated transcriptional activation of INHBA induced by methylmalonic acid promotes pancreatic neuroendocrine neoplasm progression
Abstract
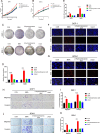
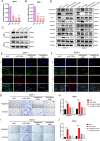
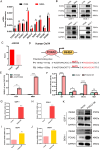
Pancreatic neuroendocrine neoplasms (PanNENs) are a group of highly heterogeneous neoplasms originating from the endocrine islet cells of the pancreas with characteristic neuroendocrine differentiation, more than 60% of which represent metastases when diagnosis, causing major tumor-related death. Metabolic alterations have been recognized as one of the hallmarks of tumor metastasis, providing attractive therapeutic targets. However, little is known about the molecular mechanism of metabolic changes regulating PanNEN progression. In this study, we first identified methylmalonic acid (MMA) as an oncometabolite for PanNEN progression, based on serum metabolomics of metastatic PanNEN compared with non-metastatic PanNEN patients. One of the key findings was the potentially novel mechanism of epithelial-mesenchymal transition (EMT) triggered by MMA. Inhibin βA (INHBA) was characterized as a key regulator of MMA-induced PanNEN progression according to transcriptomic analysis, which has been validated in vitro and in vivo. Mechanistically, INHBA was activated by FOXA2, a neuroendocrine (NE) specific transcription factor, which was initiated during MMA-induced progression. In addition, MMA-induced INHBA upregulation activated downstream MITF to regulate EMT-related genes in PanNEN cells. Collectively, these data suggest that activation of INHBA via FOXA2 promotes MITF-mediated EMT during MMA inducing PanNEN progression, which puts forward a novel therapeutic target for PanNENs.
Keywords: Epithelial–mesenchymal transition; FOXA2; INHBA; Metabolic alterations; Pancreatic neuroendocrine neoplasm; Tumor progression.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Serotonin immunoreactive pancreatic neuroendocrine neoplasm associated with main pancreatic duct dilation: a recognizable entity with excellent long-term outcome.Eur Radiol. 2021 Nov;31(11):8671-8681. doi: 10.1007/s00330-021-08007-4. Epub 2021 May 11. Eur Radiol. 2021. PMID: 33977308
-
Inhibin β-A (INHBA) induces epithelial-mesenchymal transition and accelerates the motility of breast cancer cells by activating the TGF-β signaling pathway.Bioengineered. 2021 Dec;12(1):4681-4696. doi: 10.1080/21655979.2021.1957754. Bioengineered. 2021. PMID: 34346300 Free PMC article.
-
Dual role of autotaxin as novel biomarker and therapeutic target in pancreatic neuroendocrine neoplasms.Cancer Sci. 2023 Dec;114(12):4571-4582. doi: 10.1111/cas.15980. Epub 2023 Sep 28. Cancer Sci. 2023. PMID: 37770812 Free PMC article.
-
Surgical Principles in the Management of Pancreatic Neuroendocrine Neoplasms.Curr Treat Options Oncol. 2020 Apr 30;21(6):48. doi: 10.1007/s11864-020-00736-w. Curr Treat Options Oncol. 2020. PMID: 32350693 Review.
-
The evolving (epi)genetic landscape of pancreatic neuroendocrine tumours.Endocr Relat Cancer. 2019 Aug 1;26(9):R519-R544. doi: 10.1530/ERC-19-0175. Endocr Relat Cancer. 2019. PMID: 31252410 Review.
Cited by
-
Association analysis between circulating methylmalonic acid and cognitive performance: a population-based cross-sectional study.Front Neurol. 2025 Jun 25;16:1594103. doi: 10.3389/fneur.2025.1594103. eCollection 2025. Front Neurol. 2025. PMID: 40635714 Free PMC article.
-
KDM1A-mediated ZFP64 demethylation activates CENPL to promote epithelial ovarian cancer progression.Cytotechnology. 2025 Feb;77(1):10. doi: 10.1007/s10616-024-00671-w. Epub 2024 Dec 1. Cytotechnology. 2025. PMID: 39628712
-
Molecular mechanisms and clinicopathological characteristics of inhibin βA in thyroid cancer metastasis.Int J Mol Med. 2024 Nov;54(5):99. doi: 10.3892/ijmm.2024.5423. Epub 2024 Sep 20. Int J Mol Med. 2024. PMID: 39301627 Free PMC article.
-
Metabolic changes in neuroendocrine neoplasms.Cell Mol Life Sci. 2025 May 16;82(1):205. doi: 10.1007/s00018-025-05656-2. Cell Mol Life Sci. 2025. PMID: 40377669 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

