Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer Disease Pathology
- PMID: 38252443
- PMCID: PMC10804282
- DOI: 10.1001/jamaneurol.2023.5319
Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer Disease Pathology
Abstract
Importance: Phosphorylated tau (p-tau) is a specific blood biomarker for Alzheimer disease (AD) pathology, with p-tau217 considered to have the most utility. However, availability of p-tau217 tests for research and clinical use has been limited. Expanding access to this highly accurate AD biomarker is crucial for wider evaluation and implementation of AD blood tests.
Objective: To determine the utility of a novel and commercially available immunoassay for plasma p-tau217 to detect AD pathology and evaluate reference ranges for abnormal amyloid β (Aβ) and longitudinal change across 3 selected cohorts.
Design, setting, and participants: This cohort study examined data from 3 single-center observational cohorts: cross-sectional and longitudinal data from the Translational Biomarkers in Aging and Dementia (TRIAD) cohort (visits October 2017-August 2021) and Wisconsin Registry for Alzheimer's Prevention (WRAP) cohort (visits February 2007-November 2020) and cross-sectional data from the Sant Pau Initiative on Neurodegeneration (SPIN) cohort (baseline visits March 2009-November 2021). Participants included individuals with and without cognitive impairment grouped by amyloid and tau (AT) status using PET or CSF biomarkers. Data were analyzed from February to June 2023.
Exposures: Magnetic resonance imaging, Aβ positron emission tomography (PET), tau PET, cerebrospinal fluid (CSF) biomarkers (Aβ42/40 and p-tau immunoassays), and plasma p-tau217 (ALZpath pTau217 assay).
Main outcomes and measures: Accuracy of plasma p-tau217 in detecting abnormal amyloid and tau pathology, longitudinal p-tau217 change according to baseline pathology status.
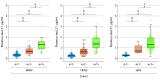
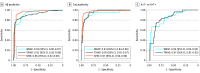
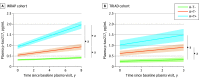
Results: The study included 786 participants (mean [SD] age, 66.3 [9.7] years; 504 females [64.1%] and 282 males [35.9%]). High accuracy was observed in identifying elevated Aβ (area under the curve [AUC], 0.92-0.96; 95% CI, 0.89-0.99) and tau pathology (AUC, 0.93-0.97; 95% CI, 0.84-0.99) across all cohorts. These accuracies were comparable with CSF biomarkers in determining abnormal PET signal. The detection of abnormal Aβ pathology using a 3-range reference yielded reproducible results and reduced confirmatory testing by approximately 80%. Longitudinally, plasma p-tau217 values showed an annual increase only in Aβ-positive individuals, with the highest increase observed in those with tau positivity.
Conclusions and relevance: This study found that a commercially available plasma p-tau217 immunoassay accurately identified biological AD, comparable with results using CSF biomarkers, with reproducible cut-offs across cohorts. It detected longitudinal changes, including at the preclinical stage.
Conflict of interest statement
Figures
References
-
- Benedet AL, Milà-Alomà M, Vrillon A, et al. ; Translational Biomarkers in Aging and Dementia (TRIAD) study, Alzheimer’s and Families (ALFA) study, and BioCogBank Paris Lariboisière cohort . Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 2021;78(12):1471-1483. doi:10.1001/jamaneurol.2021.3671 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

