Tirzepatide Improved Markers of Islet Cell Function and Insulin Sensitivity in People With T2D (SURPASS-2)
- PMID: 38252888
- PMCID: PMC11180500
- DOI: 10.1210/clinem/dgae038
Tirzepatide Improved Markers of Islet Cell Function and Insulin Sensitivity in People With T2D (SURPASS-2)
Abstract
Context: In previous SURPASS studies tirzepatide reduced hemoglobin glycated A1c (HbA1c) and body weight and improved markers of insulin sensitivity and β-cell function to a greater extent than comparators.
Objective: Explore changes in biomarkers of β-cell function and insulin sensitivity and in efficacy profiles in baseline biomarker quartile analyses with tirzepatide compared to semaglutide.
Design: Post hoc analysis of SURPASS-2 phase 3 trial (participants randomly assigned to receive weekly subcutaneous tirzepatide or semaglutide for 40 weeks).
Setting: Post hoc analysis of 128 sites in 8 countries.
Participants: A total of 1879 participants with type 2 diabetes.
Interventions: Once-weekly tirzepatide (5, 10, 15 mg) or semaglutide 1 mg.
Main outcomes measures: Change in homeostatic model assessment indices for pancreatic β-cell function (HOMA2-B) and for insulin resistance (HOMA2-IR), fasting glucagon, fasting C-peptide, and fasting insulin.
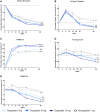
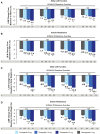
Results: At week 40, a greater increase in HOMA2-B was seen with tirzepatide (5, 10, 15 mg) doses (96.9-120.4%) than with semaglutide 1 mg (84.0%) (P < .05). There was a greater reduction in HOMA2-IR with all doses of tirzepatide (15.5%-24.0%) than with semaglutide 1 mg (5.1%) (P < .05). Tirzepatide 10 and 15 mg resulted in a significant reduction in both fasting C-peptide (5.2%-6.0%) and fasting glucagon (53.0%-55.3%) compared with an increase of C-peptide (3.3%) and a reduction of glucagon (47.7%) with semaglutide 1 mg (P < .05). HbA1c and body weight reductions were greater with all tirzepatide doses than semaglutide within each HOMA2-B and HOMA2-IR baseline quartile.
Conclusion: In this post hoc analysis, improvements in HbA1c and weight loss were consistent and significantly higher with tirzepatide, regardless of baseline β-cell function and insulin resistance, compared with semaglutide.
Keywords: beta-cell function; incretin; insulin sensitivity; tirzepatide; type 2 diabetes.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
-
- Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205‐216. - PubMed
-
- U.S. Food and Drug Administration . FDA Approves New Medication for Chronic Weight Management. https://www.fda.gov/news-events/press-announcements/fda-approves-new-med.... 2023.
-
- Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143‐155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

