Nivolumab Plus Chemotherapy in Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small-Cell Lung Cancer After Disease Progression on Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: Final Results of CheckMate 722
- PMID: 38252907
- PMCID: PMC11095864
- DOI: 10.1200/JCO.23.01017
Nivolumab Plus Chemotherapy in Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small-Cell Lung Cancer After Disease Progression on Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: Final Results of CheckMate 722
Abstract
Purpose: The phase III CheckMate 722 trial (ClinicalTrials.gov identifier: NCT02864251) evaluated nivolumab plus chemotherapy versus chemotherapy in patients with epidermal growth factor receptor (EGFR)-mutated metastatic non-small-cell lung cancer (NSCLC) after disease progression on EGFR tyrosine kinase inhibitors (TKIs).
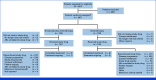
Methods: Patients with disease progression after first- or second-generation EGFR TKI therapy (without EGFR T790M mutation) or osimertinib (with/without T790M mutation) were randomly assigned 1:1 to nivolumab (360 mg once every 3 weeks) plus platinum-doublet chemotherapy (once every 3 weeks) or platinum-doublet chemotherapy alone (once every 3 weeks) for four cycles. Primary end point was progression-free survival (PFS). Secondary end points included 9- and 12-month PFS rates, overall survival (OS), objective response rate (ORR), and duration of response (DOR).
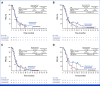
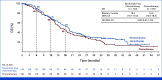
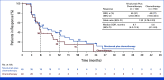
Results: Overall, 294 patients were randomly assigned. At final analysis (median follow-up, 38.1 months), PFS was not significantly improved with nivolumab plus chemotherapy versus chemotherapy (median, 5.6 v 5.4 months; hazard ratio [HR], 0.75 [95% CI, 0.56 to 1.00]; P = .0528), with 9- and 12-month PFS rates of 25.9% versus 19.8%, and 21.2% versus 15.9%, respectively. Post hoc PFS subgroup analyses showed a trend favoring nivolumab plus chemotherapy in patients with tumors harboring sensitizing EGFR mutations (HR, 0.72 [95% CI, 0.54 to 0.97]), one line of previous EGFR TKI (0.72 [95% CI, 0.54 to 0.97]), or both (0.64 [95% CI, 0.47 to 0.88]). Median OS was 19.4 months with nivolumab plus chemotherapy versus 15.9 months with chemotherapy, while ORR was 31.3% versus 26.7%, and median DOR was 6.7 versus 5.6 months, respectively. Grade 3/4 treatment-related adverse events occurred in 44.7% and 29.4% of patients treated with nivolumab plus chemotherapy and chemotherapy alone, respectively.
Conclusion: Nivolumab plus chemotherapy did not significantly improve PFS versus chemotherapy in patients with EGFR-mutated metastatic NSCLC previously treated with EGFR TKIs. No new safety signals were identified.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

