Cemiplimab for Kidney Transplant Recipients With Advanced Cutaneous Squamous Cell Carcinoma
- PMID: 38252908
- PMCID: PMC10950183
- DOI: 10.1200/JCO.23.01498
Cemiplimab for Kidney Transplant Recipients With Advanced Cutaneous Squamous Cell Carcinoma
Abstract
Purpose: Cemiplimab is approved for treating locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC). Solid organ transplant recipients have been excluded from immunotherapy trials, given concern for allograft rejection despite their increased risk of skin cancers. Chronic immunosuppression is necessary to prevent organ rejection but may attenuate antitumor response with PD-1 inhibitors.
Methods: We report a phase I study of cemiplimab for kidney transplant recipients (KTRs) with advanced CSCC. After cross-taper to a mammalian target of rapamycin (mTOR) inhibitor and pulsed dose corticosteroids (prednisone 40 mg once daily, the day before and on days 1-3 of each cycle, followed by 20 mg once daily on days 4-6, then 10 mg once daily until the day before each subsequent cycle), patients received cemiplimab 350 mg intravenously once every 3 weeks for up to 2 years and were assessed for response every 8 weeks. The primary end point was the rate of kidney rejection, with key secondary end points including rate and duration of response, and survival.
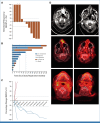
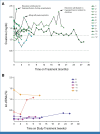
Results: Twelve patients were treated. No kidney rejection or loss was observed. A response to cemiplimab was observed in five of 11 evaluable patients (46%; 90% CI, 22 to 73), including two with durable responses beyond a year. Median follow-up was 6.8 months (range, 0.7-29.8). Treatment-related grade 3 or greater adverse events occurred in five patients (42%), including diarrhea, infection, and metabolic disturbances. One patient died of angioedema and anaphylaxis attributed to mTOR inhibitor cross-taper.
Conclusion: mTOR inhibitor and corticosteroids represent a favorable immunosuppressive regimen for KTRs with advanced CSCC receiving immunotherapy. This combination resulted in durable antitumor responses with no kidney rejection events (funded by Regeneron Pharmaceuticals [ClinicalTrials.gov identifier: NCT04339062]).
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



References
-
- Kauvar AN, Arpey CJ, Hruza G, et al. : Consensus for nonmelanoma skin cancer treatment, part II: Squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg 41:1214-1240, 2015 - PubMed
-
- Euvrard S, Kanitakis J, Claudy A: Skin cancers after organ transplantation. N Engl J Med 348:1681-1691, 2003 - PubMed
-
- Wisgerhof HC, Edelbroek JR, de Fijter JW, et al. : Subsequent squamous- and basal-cell carcinomas in kidney-transplant recipients after the first skin cancer: Cumulative incidence and risk factors. Transplantation 89:1231-1238, 2010 - PubMed
-
- Suthanthiran M, Strom TB: Renal transplantation. N Engl J Med 331:365-376, 1994 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

