An evaluation of confidence intervals for a cumulative proportion to enable decisions at interim reviews of single-arm trials
- PMID: 38253253
- PMCID: PMC10922879
- DOI: 10.1016/j.cct.2024.107453
An evaluation of confidence intervals for a cumulative proportion to enable decisions at interim reviews of single-arm trials
Abstract
Background: Clinical trials often include interim analyses of the proportion of participants experiencing an event by a fixed time-point. A pre-specified proportion excluded from a corresponding confidence interval (CI) may lead an independent monitoring committee to recommend stopping the trial. Frequently this cumulative proportion is estimated by the Kaplan-Meier estimator with a Wald approximate CI, which may have coverage issues with small samples.
Methods: We reviewed four alternative CI methods for cumulative proportions (Beta Product Confidence Procedure (BPCP), BPCP Mid P, Rothman-Wilson, Thomas-Grunkemeier) and two CI methods for simple proportions (Clopper-Pearson, Wilson). We conducted a simulation study comparing CI methods across true event proportions for 12 scenarios differentiated by sample sizes and censoring patterns. We re-analyzed interim data from A5340, a HIV cure trial considering the proportion of participants experiencing virologic failure.
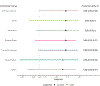
Results: Our simulation study highlights the lower and upper tail error probabilities for each CI method. Across scenarios, we found differences in the performance of lower versus upper bounds. No single method is always preferred. The upper bound of a Wald approximate CI performed reasonably with some error inflation, whereas the lower bound of the BPCP Mid P method performed well. For a trial design similar to A5340, we recommend BPCP Mid P.
Conclusions: The design of future single-arm interim analyses of event proportions should consider the most appropriate CI method based on the relevant bound, anticipated sample size and event proportion. Our paper summarizes available methods, demonstrates performance in a simulation study, and includes code for implementation.
Trial registration: ClinicalTrials.gov NCT02463227.
Keywords: Clinical trials; Clinical trials data monitoring committees; Confidence intervals; Cumulative proportion; Interim review; Single-arm trial.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The Authors declare that there are no conflicts of interest.
Figures




References
-
- Agresti A, Coull BA. Approximate Is Better than “Exact” for Interval Estimation of Binomial Proportions. The American Statistician. 1998;52(2):119–26.
-
- Brown LD, Cai TT, DasGupta A. Interval Estimation for a Binomial Proportion. Statistical Science. 2001;16(2):101–17.
-
- Clopper CJ, Pearson ES. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika. 1934;26(4):404–13.
-
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in medicine. 1998;17(8):857–72. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

