Evaluation of clinical prediction models (part 3): calculating the sample size required for an external validation study
- PMID: 38253388
- PMCID: PMC11778934
- DOI: 10.1136/bmj-2023-074821
Evaluation of clinical prediction models (part 3): calculating the sample size required for an external validation study
Abstract
An external validation study evaluates the performance of a prediction model in new data, but many of these studies are too small to provide reliable answers. In the third article of their series on model evaluation, Riley and colleagues describe how to calculate the sample size required for external validation studies, and propose to avoid rules of thumb by tailoring calculations to the model and setting at hand.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from EPSRC, NIHR-MRC, NIHR Birmingham Biomedical Research Centre, Cancer Research UK, FWO, and Internal Funds KU Leuven for the submitted work; RDR and GSC are statistical editors for The BMJ; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
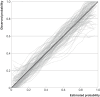


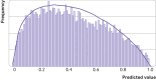
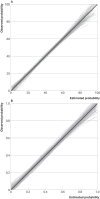
References
-
- Harrell FE, Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Springer, 2015. 10.1007/978-3-319-19425-7. - DOI
-
- Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. 2nd ed. Springer, 2019. 10.1007/978-3-030-16399-0. - DOI
-
- Riley RD, van der Windt D, Croft P, Moons KGM, eds. Prognosis Research in Healthcare: Concepts, Methods and Impact. Oxford University Press, 2019. 10.1093/med/9780198796619.001.0001. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
