Synthesis and preclinical evaluation of [11C]uPSEM792 for PSAM4-GlyR based chemogenetics
- PMID: 38253691
- PMCID: PMC10803328
- DOI: 10.1038/s41598-024-51307-0
Synthesis and preclinical evaluation of [11C]uPSEM792 for PSAM4-GlyR based chemogenetics
Abstract
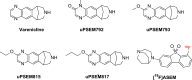
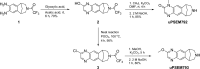
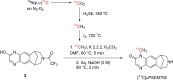
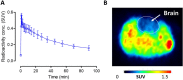
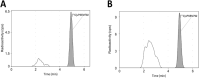
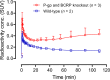
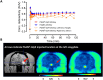
Chemogenetic tools are designed to control neuronal signaling. These tools have the potential to contribute to the understanding of neuropsychiatric disorders and to the development of new treatments. One such chemogenetic technology comprises modified Pharmacologically Selective Actuator Modules (PSAMs) paired with Pharmacologically Selective Effector Molecules (PSEMs). PSAMs are receptors with ligand-binding domains that have been modified to interact only with a specific small-molecule agonist, designated a PSEM. PSAM4 is a triple mutant PSAM derived from the α7 nicotinic receptor (α7L131G,Q139L,Y217F). Although having no constitutive activity as a ligand-gated ion channel, PSAM4 has been coupled to the serotonin 5-HT3 receptor (5-HT3R) and to the glycine receptor (GlyR). Treatment with the partner PSEM to activate PSAM4-5-HT3 or PSAM4-GlyR, causes neuronal activation or silencing, respectively. A suitably designed radioligand may enable selective visualization of the expression and location of PSAMs with positron emission tomography (PET). Here, we evaluated uPSEM792, an ultrapotent PSEM for PSAM4-GlyR, as a possible lead for PET radioligand development. We labeled uPSEM792 with the positron-emitter, carbon-11 (t1/2 = 20.4 min), in high radiochemical yield by treating a protected precursor with [11C]iodomethane followed by base deprotection. PET experiments with [11C]uPSEM792 in rodents and in a monkey transduced with PSAM4-GlyR showed low peak radioactivity uptake in brain. This low uptake was probably due to high polarity of the radioligand, as evidenced by physicochemical measurements, and to the vulnerability of the radioligand to efflux transport at the blood-brain barrier. These findings can inform the design of a more effective PSAM4 based PET radioligand, based on the uPSEM792 chemotype.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

