HDAC1/2 control mesothelium/ovarian cancer adhesive interactions impacting on Talin-1-α5β1-integrin-mediated actin cytoskeleton and extracellular matrix protein remodeling
- PMID: 38254102
- PMCID: PMC10804625
- DOI: 10.1186/s13046-023-02930-8
HDAC1/2 control mesothelium/ovarian cancer adhesive interactions impacting on Talin-1-α5β1-integrin-mediated actin cytoskeleton and extracellular matrix protein remodeling
Abstract
Background: Peritoneal metastasis, which accounts for 85% of all epithelial ovarian carcinoma (EOC) metastases, is a multistep process that requires the establishment of adhesive interactions between cancer cells and the peritoneal membrane. Interrelations between EOC and the mesothelial stroma are critical to facilitate the metastatic process. No data is available so far on the impact of histone acetylation/deacetylation, a potentially relevant mechanism governing EOC metastasis, on mesothelial cells (MCs)-mediated adhesion.
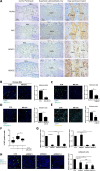
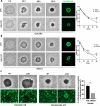
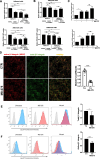
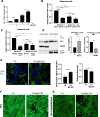
Methods: Static adhesion and peritoneal clearance experiments were performed pretreating mesenchymal-like MCs and platinum-sensitive/resistant EOC cell lines with MS-275-a Histone deacetylase (HDAC)1-3 pharmacological inhibitor currently used in combination trials. Results were acquired by confocal microscopy and were analyzed with an automated Opera software. The role of HDAC1/2 was validated by genetic silencing. The role of α4-, α5-α1 Integrins and Fibronectin-1 was validated using specific monoclonal antibodies. Quantitative proteomic analysis was performed on primary MCs pretreated with MS-275. Decellularized matrices were generated from either MS-275-exposed or untreated cells to study Fibronectin-1 extracellular secretion. The effect of MS-275 on β1 integrin activity was assessed using specific monoclonal antibodies. The role of Talin-1 in MCs/EOC adhesion was analyzed by genetic silencing. Talin-1 ectopic expression was validated as a rescue tool from MS-275-induced phenotype. The in vivo effect of MS-275-induced MC remodeling was validated in a mouse model of peritoneal EOC dissemination.
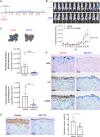
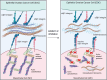
Results: Treatment of MCs with non-cytotoxic concentrations of MS-275 caused a consistent reduction of EOC adhesion. Proteomic analysis revealed several pathways altered upon MC treatment with MS-275, including ECM deposition/remodeling, adhesion receptors and actin cytoskeleton regulators. HDAC1/2 inhibition hampered actin cytoskeleton polymerization by downregulating actin regulators including Talin-1, impairing β1 integrin activation, and leading to abnormal extracellular secretion and distribution of Fibronectin-1. Talin-1 ectopic expression rescued EOC adhesion to MS-275-treated MCs. In an experimental mouse model of metastatic EOC, MS-275 limited tumor invasion, Fibronectin-1 secretion and the sub-mesothelial accumulation of MC-derived carcinoma-associated fibroblasts.
Conclusion: Our study unveils a direct impact of HDAC-1/2 in the regulation of MC/EOC adhesion and highlights the regulation of MC plasticity by epigenetic inhibition as a potential target for therapeutic intervention in EOC peritoneal metastasis.
Keywords: Actin cytoskeleton; Epithelial ovarian Cancer; Extracellular matrix; Fibronectin-1; HDAC1–2; Integrin; MS-275; Mesothelial to mesenchymal transition (MMT); Peritoneal Carcinomatosis; Peritoneum; Talin1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. - DOI - PubMed
-
- Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK collaborative trial of ovarian Cancer screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–956. doi: 10.1016/S0140-6736(15)01224-6. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

